The research, described in Nature Cell Biology, provides the first evidence that dynamic actin polymerisation in the nucleus helps in resizing the nucleus along with reorganising the genome after cell division (mitosis). Principal Investigator Prof Robert Grosse says “this discovery advances our fundamental knowledge of the functions of nuclear actin dynamics as well as genome regulation in space and time, and could have major implications in understanding cancer and regeneration.”
In mammals, including humans, the cell nucleus serves as an organelle for intricate packaging and protection of the genome. When human cells divide (a process known as mitosis), the nucleus gets dissembled to allow the highly-compacted genome (chromosomes) to be accurately segregated to the daughter cells. Once chromosome segregation is completed, the new cells need to re-establish the nuclear compartments and de-compact these chromosomes. These post-mitosis processes, although essential for life, were poorly understood.
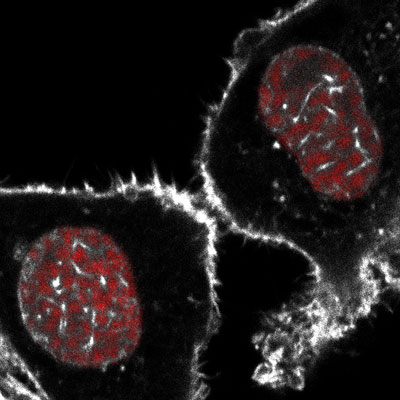
Figure: Image taken from live cell recordings of dividing fibroblasts captured during early G1 of the cell cycle. Cellular actin as well as nuclear actin filaments are shown in white using a shuttling anti-actin Chromobody and chromatin is shown in red using H2B-mCherry.
An unusual suspect
In this work, Prof Robert Grosse’s laboratory (University of Marburg, Germany) revealed the formation of transient and highly dynamic F-actin in the nucleus of daughter cells as they start rebuilding their nuclei after mitosis. The polymerisation of actin (F-actin) readily occurs in the cytoplasm of cells where it serves a very important function in controlling cell shape and enabling cells to crawl around. Discovering this transient and dynamic F-actin in the nucleus soon after cell division hinted that it may be required for rebuilding the nucleus and re-organising the genome. To investigate this, the research teams of Robert Grosse, Abderrahmane Kaidi (University of Bristol, UK) and Kei Miyamoto (Kindai University, Japan) developed and implemented complementary and interdisciplinary methods to visualise nuclear structure and genome organisation after cell division. In so doing, they revealed that disruption of the formation of this F-actin results in cells failing to expand their nuclear volume as well as their inability to de-compact their genome. Because of these defects, cells become inefficient in retrieving genetic information encoded in their DNA; thus, they divide slower, which also impacts on early mammalian development.
“This research highlights the importance of the spatiotemporal control of nuclear organisation for normal cells’ function, and we continue to define the principals that regulate these processes and their potential impact on cancer and regeneration.
"This collaborative study, supported by HFSP, enables a wonderfully fruitful and timely synergy between the three research teams,“ Robert Grosse concludes.
Reference
A transient pool of nuclear F-actin at mitotic exit controls chromatin organization. Christian Baarlink, Matthias Plessner, Alice Sherrard, Kohtaro Morita, Shinji Misu, David Virant, Eva-Maria Kleinschnitz, Robert Harniman, Dominic Alibhai, Stefan Baumeister, Kei Miyamoto, Ulrike Endesfelder, Abderrahmane Kaidi & Robert Grosse. Nature Cell Biology 19, 1389–1399 (2017). doi:10.1038/ncb3641.


































