Over 90% of abdominal surgeries result in adhesions, a third of which end in re-hospitalization within 10 years, creating an enormous healthcare burden, estimated at over $1 billion a year in the United States alone. The mechanisms leading injured organ surfaces to seam remains undisclosed, primarily because of a lack of unidentifiable cellular origin.
Here, we discover that adhesions develop from the epithelial lining of the internal organs, called mesothelium. Using clonal analysis and lineage tracing, we discovered that the surface mesothelium which lines all of the internal organs, is the cellular source of fibrous adhesions. RNA sequencing and bioinformatics analyses of mouse mesothelial cells from injured mesothelium revealed aspects of the pathological mechanism of adhesion development and yielded several potential regulators of this process. We found that we can inhibit adhesions in mouse models by targeting mesothelial regulatory molecules that are upregulated during adhesion formation, such as hypoxia inducible factor 1 alpha (HIF1α), Mesothelin and CD47. Analyses of human adhesion tissue suggested that similar signaling pathways may contribute to surgical adhesions in human patients.
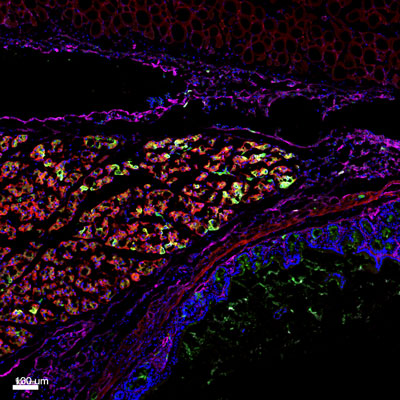
Figure: Histologic section of an adhesion site between the peritoneum and intestine, in mice. Using a mouse model of post-operative adhesions in combination with lineage tracing techniques, we determined that mesothelium lining the organs (magenta) is the cellular source of adhesion tissue.
Prevention agents to universally reduce surgical adhesions in oncology, and gynecological, pelvic and abdominal surgery are urgently needed and do not show significant efficacy in clinical trials, primarily because of elusive pathomechanisms. Our findings provide potential new therapeutic avenues to treat post-operative adhesions across a range of surgical arenas.


































