Biological rhythms are a widespread characteristic of life and enable organisms to coordinate their biological processes with the predictable cycles of their environment, food, predators and, in the case of parasites, their hosts. Malaria causing parasites (Plasmodium spp.) exhibit daily rhythms in their development inside their host’s red blood cells. This causes the regular fevers characteristic of the disease in humans. How and why malaria parasites replicate rhythmically in the blood are long standing mysteries.
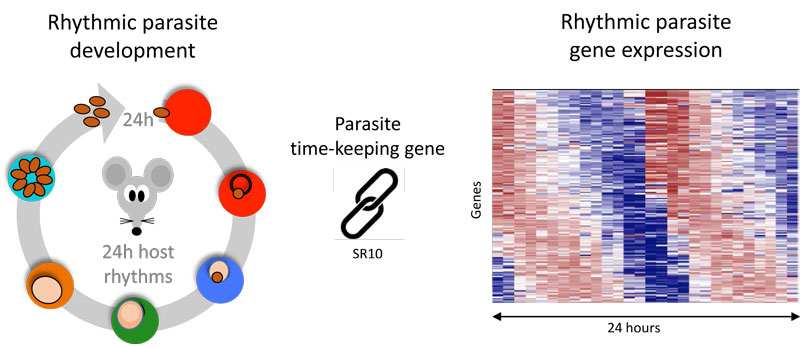
Figure: Malaria parasite development inside red blood cells is rhythmic, leading to daily cycles of replication. SR10 allows parasites to coordinate (synchronise) their timing with rhythms in their mammalian host.
A new genetic analysis revealed that timing matters for essential cellular processes in malaria parasites and reveals some of the internal timekeeping systems that schedule rhythms in parasite development. Researchers from the University of Edinburgh (UK), King Abdullah University of Science and Technology (KAUST, Saudi Arabia) and Nagasaki University (Japan) teamed up to study gene activity patterns in mouse-infecting malaria parasites. They found that more than half of all the parasite’s genes exhibited 24-hour cycles of activity, ramping up and down at regular daily intervals. Around half of the rhythmic genes lost their periodic activity when the rhythms of the host and parasite host fell out of synchrony, impairing important cellular processes for the parasite.
Human malaria parasites, that have a 48 hour developmental rhythm, cultured without environmental time-cues also displayed daily cycles of activity in gene expression. One such rhythmic gene coded for the SR10 protein in both mouse and human parasites and was found to act as a cog in the parasite’s internal time-keeping machinery. Without this protein, the usual 24-hour cycle of the mouse parasite became shorter, leading to defective cellular processes that were also affected when parasite and host rhythms were out of synch.
The knowledge from this study may inform new therapies for malaria treatment. This information might allow doctors to formulate drug regimens in which patients take anti-malarial therapies with known target genes at particular times of the day so as to eliminate the malaria parasite more effectively.
by Aidan O'Donnell, University of Edinburgh


































