The enteric nervous system (ENS) contains over 100 million nerve cells called enteric neurons, which have important roles in intestinal physiology, including peristalsis. The microbiota has emerged as a critical regulator of ENS functions. For example, germ-free (GF) mice are characterized by reduced enteric neuronal excitability, which in turn leads to changes in gut motility and prolonged intestinal transit time. However, colonization of adult GF mice with a normal microbiota restores neuronal excitability and ENS motor functions, suggesting that intestinal neural circuits are endowed with molecular machinery that monitors the state of the gut lumen and adjusts neuronal activity and motility accordingly.
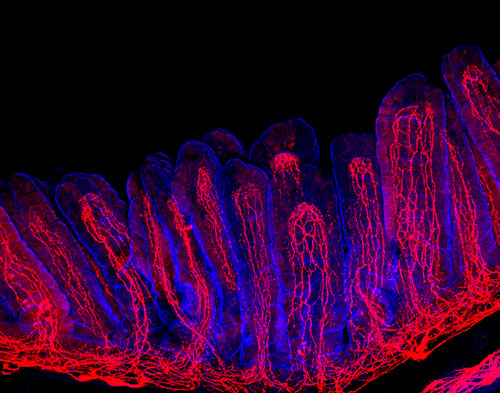
Figure 1: Intestinal villi (blue) with neuronal fibers (red). Copyright: Yuuki Obata, The Francis Crick Institute.
We hypothesized that molecular mechanisms linking microbiota to ENS functions are encoded by genetic programs operating predominantly in neural circuits of the colon, the intestinal segment with the heaviest microbial load. This led us to perform RNA sequencing to identify genes specifically upregulated in colonic neurons in response to microbial colonization. Transcriptome profiling of enteric neurons that represent distinct intestinal segments and microbiota states identified several genes specifically expressed in colonic neurons in a microbiota-dependent manner. Among them was the gene encoding a transcription factor, Aryl hydrocarbon Receptor (AhR). AhR is well known as an environmental sensor, which is activated by a broad range of microbial, dietary and endogenous metabolites, as well as environmental toxins (AhR ligands).
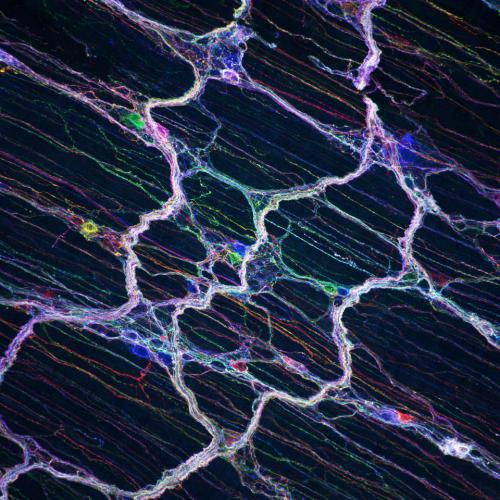
Figure: Myenteric plexus labelled with multiple fluorescent proteins (Enterainbow). Copyright: Yuuki Obata, The Francis Crick Institute.
In order to understand the physiological role of AhR in enteric neurons, we generated two independent transgenic mouse models with enteric neuron-specific ablation of AhR signaling, deletion of AhR (AhREN-KO mice) and constitutive activation of AhR ligand metabolism (ENCyp1a1 mice). Interestingly, both animals showed increased intestinal transit time, indicating that activation of neuronal AhR signaling by its ligands is required for normal gut motility. In fact, dietary delivery of the compound Indole-3-carbinol, which generates high-affinity AhR ligands, rescued the gut dysmotility in ENCyp1a1 animals. Although depletion of microbiota results in delayed gut transit, similar to that observed in AhREN-KO mice, overexpression of AhR in enteric neurons of microbiota-depleted mice partially rescued gut dysmotility. These data provide direct evidence that neuron-intrinsic AhR signaling is implicated in the regulation of gut motility by microbiota.
In summary, this study identified a novel molecular mechanism that allows neural circuits to directly monitor and respond to changes in the gut microenvironment. Given that peristalsis is an important defensive mechanism of the gut, AhR signaling may serve as a neuron-controlled surveillance pathway that promotes propulsion of luminal contents in response to abnormal bacterial overgrowth or dysbiosis. This study helps us understand the common gastrointestinal motility disorders such as constipation and irritable bowel syndrome (IBS) and may lead to the development of novel therapeutic strategies based on pharmacological activation of AhR, dietary intervention or microbiota transplantation.


































