We live in a rhythmic world, and so do the parasites that live inside us. As human beings, we follow a very repeatable pattern every day: sleep, work, eat, repeat... Most of our biological processes also follow daily rhythms. This includes rhythms in activity, body temperature, food intake, metabolism, immune function and urine production to name a few. To know what to do, and when to do things each day, we take cues from our environment, primarily the sun, to set the timing of our internal clocks. We need these “circadian” clocks for successful survival and reproduction because they allow us to coordinate our biological processes with rhythms in our environment.
Figure 1: Image of Plasmodium chabaudi inside red blood cells.
Parasites also live in a rhythmic world inside their hosts because they are subjected to their host’s daily rhythms and several studies have begun to discover how host rhythms affect parasites (e.g. Rijo-Ferreira et al. 2017). Our lab has published a new study demonstrating that the timing of host’s meals determines the timing of rhythms in replication by their malaria parasites.
HFSP funding was a fundamental step to getting this project off the ground, which used a rodent model system to show that when the mice’s mealtime changed, the parasites living inside them altered the timing of infecting red blood cells and undergoing mitosis.
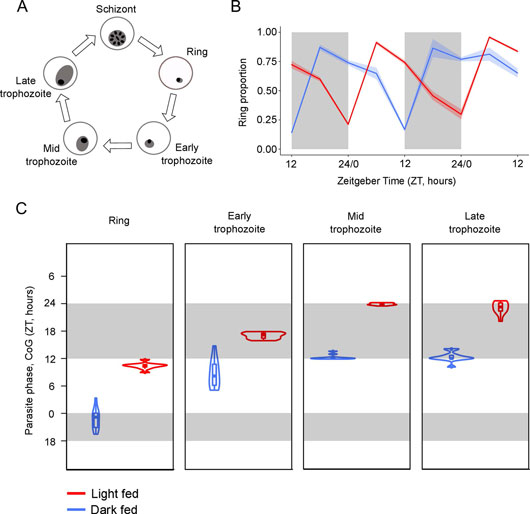
Figure 2: Parasite rhythms are inverted in hosts fed during the day compared to the night. (A) The asexual cycle of malaria parasites is characterised by five morphologically distinct developmental stages (ring, early trophozoite, mid trophozoite, late trophozoite) differentiated by parasite size within the red blood cell, the size and number of nuclei, and the appearance of haemozoin. (B) Mean ± SEM (N = 10 per group) proportion of observed parasites in the blood at ring stage in light fed mice (red; allowed access to food during the day, between ZT 0 and ZT 12) and dark fed mice (blue; allowed access to food during the night, between ZT 12 and ZT 24). The proportion of parasites at ring stage in the peripheral blood is highest at night (ZT 22) in dark fed mice but in the day (ZT 10) for light fed mice, illustrating the patterns observed for all other (rhythmic) stages. (C) CoG (estimate of phase) in ZT (h) for each rhythmic parasite stage in the blood. Each violin illustrates the median ± IQR overlaid with probability density (N = 10 per group). The height of the violin illustrates the variation in the timing of the CoG between mice and the width illustrates the frequency of the CoGs at particular times within the distribution. Sampling occurred every 6 hours days 6-8 post infection. Light and dark bars indicate lights on and lights off (lights on: ZT 0, lights off: ZT 12).
Alternatively, parasites might be using host feeding as an environmental time cue, just as we use the sun to time our rhythms. If certain parasite stages during replication are more susceptible to rhythmic immune responses at certain times of day, parasites should schedule their rhythm to avoid the most damaging immune attack. However, it is thought that parasites do not organise their rhythms but that hosts use their rhythmic immune responses to generate rhythms in parasites. Our experiments overturn this notion. By measuring host cytokines during infections as a marker of the host immune response, we were able to reveal that the parasites themselves generate a rhythmic immune response. This means that immune rhythms are a product of the parasites’ timing, not a cause of their timing.Why might eating be important for parasite rhythms? We followed up our observation and found that increasing blood glucose concentration, associated with mealtimes, coincided with the time at which the most metabolically active parasite forms are present in the blood. This suggests that parasites are using glucose as a food source and cannot complete their development without it.
Understanding what drives the timing of parasite rhythms is important. Like us, parasites get jet lag, suffering a cost in numbers when the timing of their rhythms is made out-of-sync with the timing of their hosts (O’Donnell et al. 2011). Thus, interfering with parasite rhythms reveals opportunities to reduce the severity of infection and transmission to new hosts. Disrupting the link between eating and parasite timing – perhaps through host diet, or via drugs that manipulate the biological pathways involved in time-keeping – could reduce both the severity and spread of malaria infection.
We have studied malaria parasites, but rhythms matter to other parasites too, for example, in transmitting to vectors (Hawking 1967) and establishing infections in new hosts (Bellet et al. 2013; Shirasu-Hiza et al. 2007; Roden and Ingle 2009). With increasing reports of drug and insecticide resistance, the hunt is on for new cures and new ways to stop transmission. Knowing how differences in timing impact both parasites and hosts, as well as understanding the biological mechanisms controlling their rhythms, will give us the tools to be better able to tackle many types of infection.
Text by Kim Prior and Sarah Reece
Reference
Timing of host feeding drives rhythms in parasite replication. 2018. Prior KF, van der Veen DR, O’Donnell AJ, Cumnock K, Schneider D, Pain A, Subudhi A, Ramaprasad A, Savill NJ, Rund SSC, Reece SE. PLoS Pathog, 14:e1006900. https://doi.org/10.1371/journal.ppat.1006900.
Other references
Circadian clock regulates the host response to Salmonella. Bellet MM, Deriu E, Lui JZ, Grimaldi B, Blaschitz C, Zeller M, Edwards RA, Sahar S, Dandekar S, Baldi P, George MD, Raffatellu M, Sassone-Corsi P. 2013. PNAS, 110, 9897-9902.
The 24-hour periodicity of microfilariae: biological mechanisms responsible for its production and control. 1967. Hawking F. Proc R Soc B, 169, 59-76.
Fitness costs of disrupting circadian rhythms in malaria parasites. O’Donnell AJ, Schneider P, McWatters HG, Reece SE 2011. Proc R Soc B, 278, 2429-2436.
Trypanosoma brucei metabolism is under circadian control. Rijo-Ferreira F, Pinto-Neves D, Barbosa-Morais NL, Takahashi JS, Figueiredo LM. 2017. Nat Microbiol, 13, 17032.
The role of light and the circadian clock in determining the outcome of plant-pathogen interactions. Roden LC, Ingle RA. Lights, rhythms, infection: 2009. The Plant Cell, 21, 2546-2552.


































