During embryonic development tissues remodel their form. This is achieved via cell shape changes that allow a tissue to fold, shrink or elongate, thin or thicken. While much work has been done to reveal how a tissue can remodel its form by understanding how its constituting cells change shape, little is known on how remodeling tissues interact and how the properties and the mechanics of cells are integrated at the embryo scale to allow tissue coordination during development. In this study we use the fruit fly Drosphila embryo as a model system to study tissue fold formation. Tissue folding is an important topological change since it allows the translocation of cells towards the inside of the embryo where inner organs will eventually be formed: this process is known as gastrulation. During gastrulation in the fly the prospective mesoderm, which is located on the ventral side, folds and becomes internalized (mesoderm invagination). At the same time the lateral tissues (the ectoderm) and the dorsal tissue (the amnioserosa) displace and elongate. How do mesoderm, ectoderm and amnioserosa tissues interact during gastrulation and how is their behavior controlled and coordinated in time?
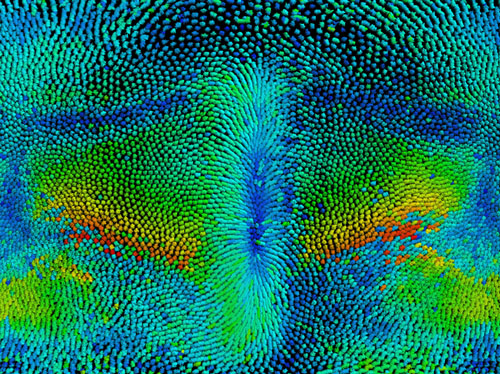
Figure: Cylindrical projection of cell tracks over the entire embryo during the first steps of fruit fly development. The embryo anterior part is at the top, posterior at the bottom, ventral in the middle and dorsal at the left and right side. Warmer colors represent higher cell speed displacement taken at a given time point.
To correlate tissue behavior at different sites of the embryo we devised and used a light sheet microscope with dual detection (MuVi-SPIM) to image the embryo in 3D over time at high spatial and temporal resolution. We used optical cross-sections and a cylindrical projection of the entire embryo to track and characterize the shape of cell apical surface for wild type (see Figure) and mutated embryos. We equipped the light sheet microscope with a femtosecond infra-red laser capable of locally cauterizing the epithelial tissue and generating fixed boundaries that impair tissue displacements.
Our study shows that ectoderm displacement is necessary for mesoderm invagination. The left and right ectodermal sheets are mechanically coupled and work in tandem to define the ventral midline position. The apical acto-myosin scaffolds of mesoderm, ectoderm and amnioserosa cells are distinct during this process. A computational model that recapitulates all three tissue behaviors suggests that the tissues have different mechanical properties; experimental analysis confirms these properties in vivo. Our study shows how different mechanical properties of tissues are integrated at the embryo scale, which is a key step to understanding the logic of embryo morphology.
Reference
Embryo-scale tissue mechanics during Drosophila gastrulation movements. M. Rauzi, U. Krzic, T. E. Saunders, M. Krajnc, P. Ziherl, L. Hufnagel, M. Leptin, Nature communications 6, (2015); published online Epub10/26/online (10.1038/ncomms9677).


































