Branching morphogenesis is a developmental mechanism used to establish the basic architecture of many organs. It mediates the elaboration of epithelial tubes in organ primordia into the tree-like structures that make up the airways of the lungs, the ducts of glandular structures and the urine collecting network in the kidney. These networks are central to establishing platforms for exchange, secretion and excretion. The observation that branching morphogenesis is a feature of multiple organs in different organisms raises the possibility that it occurs through a unifying mechanism and a recent study has suggested that this is the case (Hannezo et al. 2017). Examination of mammary gland development provided strong evidence that branching ceases stochastically due to the physical proximity of the tips of growing tubes to neighboring branches. This notion of stochastic cessation of branching was then extended to the kidney, where nephron formation was proposed as an event which halts the branching process. The results were used to propose a unifying theory of branching morphogenesis. Our previous HFSP funded studies had profiled nephron formation and cellular dynamics across developmental time (Short et al. 2014, Combes et al. 2014) and our results seemed at odds with such a theory. On this basis we then examined the impact of nephron formation on the process of renal branching morphogenesis (Short et al. 2018).
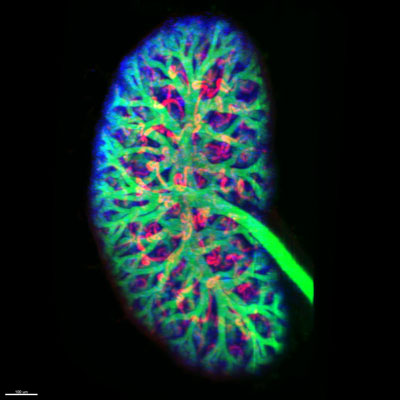
Figure: A fetal mouse kidney labelled with antibodies to detect the collecting ducts (green), nephrons (red) and proliferating cells (blue) imaged using a light sheet microscope. Image credit: Dr Kieran Short, Monash University.
Link to video (source Elife: Branching morphogenesis in the developing kidney is not impacted by nephron formation or integration. Short et al., Elife. 2018 Jul 31;7. pii: e38992.)
Nephrons form from a pool of mesenchymal progenitor cells closely associated with the tips of the branching renal epithelium. As development progresses, sub-populations of these cells undergo a transformation to generate epithelial renal vesicles, which then elaborate to form nephrons and reintegrate back into the branching epithelium. In our study of mouse kidney development, we found no evidence for a relationship between nephron differentiation and the branching process – tips with attached nephrons continued to branch as development progressed. Moreover, we found no evidence that the presence of nephrons affected the proliferation of progenitor cells in either the tips of the branching ureteric epithelium or in the progenitor cell populations from which they derive. Taken together these results indicate very strongly that branching morphogenesis in the mammary gland and kidney does not progress in a similar fashion and that nephron formation is not a trigger for cessation of branching in the kidney. Although branching morphogenesis is a common feature of organogenesis, it would appear unlikely to be mediated through a “unifying” developmental mechanism.
HFSP funding was central to allowing us to develop approaches to model renal branching morphogenesis and it helped us to develop imaging methods to examine the cellular and whole-tissue events that drive renal development (Short et al. 2014, Combes et al. 2014). The approaches developed with HFSP funding have now been applied to the characterization of a growing number of mutant lines with kidney defects, revealing a variety of ways in which perturbation of the branching epithelium or the nephron progenitor population can be affected (Volovelsky et al. 2018, Combes et al. 2017, Lefevre et al. 2017, Wainwright et al. 2015).
Reference
Branching morphogenesis in the developing kidney is not impacted by nephron formation or integration. Short KM, Combes AN, Lisnyak V, Lefevre JG, Jones LK, Little MH, Hamilton NA, Smyth IM. Elife. 2018 Jul 31;7. pii: e38992. doi: 10.7554/eLife.38992.
Other references
A Unifying Theory of Branching Morphogenesis. Hannezo E, Scheele CLGJ, Moad M, Drogo N, Heer R, Sampogna RV, van Rheenen J, Simons BD. Cell. 2017 Sep 21;171(1):242-255.e27.
Global quantification of tissue dynamics in the developing mouse kidney. Short KM, Combes AN, Lefevre J, Ju AL, Georgas KM, Lamberton T, Cairncross O, Rumballe BA, McMahon AP, Hamilton NA, Smyth IM, Little MH. Dev Cell. 2014 Apr 28;29(2):188-202.
Hamartin regulates cessation of mouse nephrogenesis independent of Mtor. Volovelsky O, Nguyen TN, Combes AN, Wilson S, Little MH, Witte DP, Brunskill EW, Kopan R. Proc. Natl. Acad. Sci. (2018) 115(23): 5998-6003.
Branching morphogenesis in the developing kidney is governed by rules that pattern the ureteric tree. Lefevre JG, Short KM, Lamberton TO, Michos O, Graf D, Smyth IM, Hamilton NA. Development. 2017 Dec 1;144(23):4377-4385.
Haploinsufficiency for SIX2 increases nephron progenitor proliferation leading to elevated branching and nephron number. Combes A, Wilson S, Phipson B, Binnie B, Ju A, Lawlor K, Cebrian C, Walton S, Smyth I, Moritz K, Kopan R, Oshlack A, and Little MH. Kid Int. (2017) 93(3): 589-598.
ROBO2 restricts the nephrogenic field and regulates Wolffian duct–nephrogenic cord separation. Wainwright EN, Wilhelm D, Alexander N, Combes A, Little MH, Koopman MH. Dev. Biol (2015) 404(2): 88-102.
An integrated pipeline for the multidimensional analysis of branching morphogenesis. Combes AN, Short KM, Lefevre J, Hamilton NA, Little MH, Smyth IM. Nat Protoc. 2014 Dec;9(12):2859-79.


































