 |
Takashi Fukaya obtained his PhD from the University of Tokyo studying the mechanism of gene silencing by microRNAs in the lab of Yukihide Tomari. He then carried out postdoctoral research with Michael Levine at UC Berkeley and Princeton University from 2014 to 2018, when he was supported by an HFSP Long-Term Fellowship. In 2018, he started his own lab at the Institute for Quantitative Biosciences at the University of Tokyo. |
The nature of regulatory interaction between distal enhancers and target promoters has been a long-standing mystery in gene regulation. In this study, we exploited the properties of transvection to explore enhancer-promoter communication between homologous chromosomes in living Drosophila embryos.
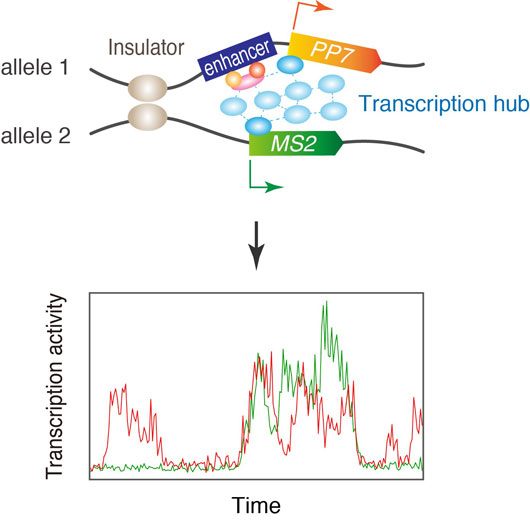
Figure: Model for gene activation via transcription hub.
Using the MS2 live imaging method, we successfully visualized transvection in living Drosophila embryos. A well-defined developmental enhancer was placed in trans to a lacZ reporter gene containing a series of MS2 stem loops, permitting detection of nascent transcripts using an MCP-GFP fusion protein (Garcia et al., 2013, Lucas et al., 2013). We found that trans-activation of the MS2 reporter gene relies on a pair of insulator DNAs located in both alleles. Different classes of insulators display distinct orientation requirements and differential kinetics of trans-activation. Heterotypic pairs of insulators failed to mediate transvection, suggesting that insulator specificity underlies higher-order chromosomal organization and long-range enhancer-promoter interaction.
An unresolved mystery of transvection is the relative levels of expression of the cis and trans transcription units when regulated by a shared enhancer. Does the enhancer on one homolog have equal access to target genes located on both homologs? To address this question, we employed newly developed dual-fluorescent MS2/PP7 live imaging (Fukaya et al., Cell 2016) to examine if a single enhancer can co-activate a cis-linked PP7 reporter gene together with the MS2 reporter gene in trans. Surprisingly, we observed a high incidence of co-activation and tight association of the two reporter genes, suggesting that the two alleles share a common pool of transcription apparatus during transvection.


































