The transformation of the morula-stage embryo into a blastocyst is a fundamental process during preimplantation mammalian development. To undergo this transition, the cells of the embryo must first establish a permeability barrier. This epithelial barrier seals the embryo from the exterior to enable expansion of the first embryonic cavity, but the mechanism triggering embryo sealing is not known.
Here, we discover a three-stage actin zippering process that enables junctional maturation and is required to seal the embryo for blastocyst formation.
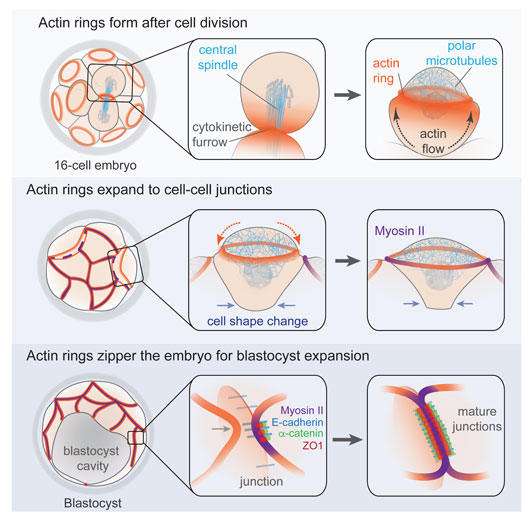
Figure: Schematic summary of the main events driving actin ring formation, expansion, and zippering along cell-cell junctions in the mouse embryo.
First, an actin ring forms at the apical cortex of cells following division in the morula stage embryo. We demonstrate that the actin ring forms de novo via the combined action of actin flows and a network of microtubules that clears F-actin from the cortex inside the ring, in an analogous manner to polar relaxation in other systems.
Second, the actin rings expand and couple to cell-cell junctions. Unlike well-characterized actin rings that drive morphogenetic processes such as cytokinetic scission or wound healing by myosin II-dependent constriction, the actin rings of the mouse embryo have negligible levels of myosin II. Instead of constricting, they expand over the entire apical cortex to the cell-cell junctions. Once there, they recruit key components of adherens and tight junctions, which couple the rings to the junction and trigger a localized accumulation of myosin II.
Third, a tension-dependent mechanism zippers the actin rings of neighbouring cells along the junctions, forming a mature junctional belt and establishing the permeability barrier. Successful completion of the zippering process is required for the embryo to withstand increases in internal pressure during formation of the blastocyst cavity.
Together, our findings reveal a coordinated morphogenetic mechanism comprising actin ring formation, expansion, and zippering, which seals the embryo to allow the critical transition from a morula into a blastocyst.
Reference
Expanding Actin Rings Zipper the Mouse Embryo for Blastocyst Formation. Zenker, J.*, White, MD.*, Gasnier, M*., Alvarez, YD.*, Lim HYG., Bissiere, S, Biro, M., Plachta, N. Cell (2018), DOI: https://doi.org/10.1016/j.cell.2018.02.035.


































