This study by Baccelli et al. reveals that a large subgroup of chemotherapy-resistant AMLs is addicted to oxidative phosphorylation and can thus be successfully treated using inhibitors of the mitochondrial electron transport chain.
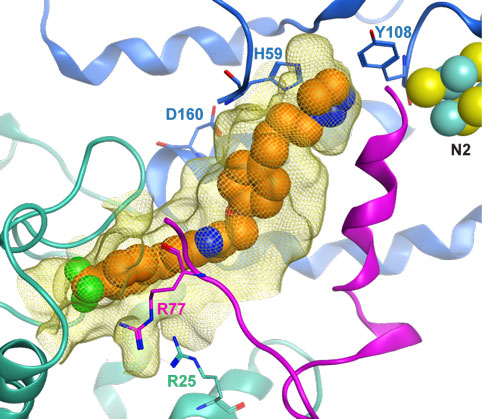
Figure: Hypothetical binding model of mubritinib (shown as orange spheres) in the ubiquinone-binding pocket of the Electron Transport Chain complex I (highlighted as a yellow mesh), built in the mammalian complex (PDB entry 5LC5). Subunits 49kDa, PSST and ND1 are shown as blue, purple and green ribbons, respectively. Selected side-chains lining the site are depicted as sticks and Fe-S cluster N2 is shown as spheres.
Standard treatment for AML includes a combination of cytarabine and anthracycline as an induction regimen, followed by consolidation chemotherapy or hematopoietic stem cell transplantation, depending on the patient’s genetic risk class. Although 60% to 70% of patients enter complete remission after the induction regimen, most of them relapse within 3 years due to the outgrowth of resistant cells. Thus, the identification of novel treatment strategies, in particular for chemotherapy-resistant patients, is a pressing issue.
Baccelli et al. found that a small molecule Mubritinib is selectively effective in vitro against a large number of poor outcome leukemias (estimated around 25,000 new cases per year in western countries). Surprisingly, the authors found that the small molecule does not exert its antileukemic activity through its putative target (ERBB2, a receptor tyrosine kinase), but rather through the direct and ubiquinone-dependent inhibition of the mitochondrial electron transport chain (ETC) complex I. Indeed, sensitive leukemias were found to be oxidative phosphorylation (OXPHOS) hyperactive, making them highly vulnerable to ETC inhibitors.
While upregulation of glycolysis, one of the metabolic reprogramming processes of tumor cells, has been extensively investigated as a potential therapeutic target, the dependence on mitochondrial OXPHOS is still in the early stages of exploration. In the case of blood cancers, the approach appears particularly attractive as it has recently been demonstrated that healthy hematopoietic stem cells are mostly dependent on glycolysis rather than OXPHOS for their energy supply. Accordingly, in vivo treatment of mice bearing poor outcome human leukemias with Mubritinib successfully delayed the development of the disease, without toxic side effect in animals.
A license for the patents that were created based on our findings has been bought by a large pharmaceutical company, in order to develop novel treatments for poor outcome AML.


































