Tissues, cells, and intracellular structures need to have the right size to function properly. A remarkable example of size regulation is how the mitotic spindle, which segregates the genetic material to the daughter cells, adapts to cell size during the first rounds of cell division in a developing embryo. In general, smaller cells have smaller spindles, but in very large cells the size of spindles reaches an upper limit. What sets this upper size limit is still not understood. Furthermore, the main building blocks of the spindle, the microtubules, only last for tens of seconds while the entire structure remains for minutes or even up to several hours. This requires constant creation of new microtubules, a process that is called microtubule nucleation. Up to now, it has been elusive how microtubule nucleation is regulated in spindles.
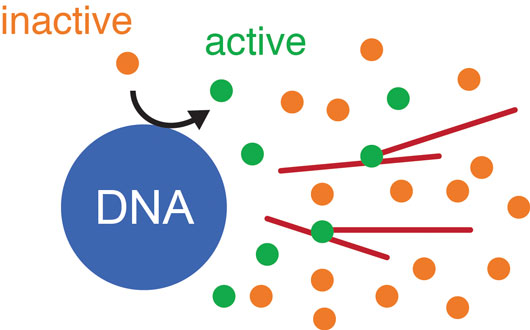
Figure: Schematic drawing of the mathematical model for microtubule nucleation in spindles. Inactive nucleators (orange) are activated at the DNA (blue). These active nucleators (green) diffuse away from the DNA and bind and unbind microtubules (red). Once bound, they can nucleate new microtubules with a certain probability. Active nucleators are inactivated at a constant rate.
To investigate the mechanisms of microtubule nucleation in spindles and their relation to spindle size, we developed a new method based on laser ablation to detect nucleation sites in dense spindle structures assembled in Xenopus laevis egg extracts and found that nucleation is autocatalytic and spatially regulated. By combining this method with biochemical perturbations and mathematical modeling, we showed that new microtubules grow off the side of pre-existing microtubules. This self-amplification is regulated by a gradient of nucleators emanating from the DNA in the center of the spindle. This means the farther away from the DNA a microtubule is, the less likely it is that this microtubule will nucleate a new one, which ultimately limits the size of the spindle.
Our results shed light on the mechanisms regulating spindle size. We propose that spindle size is controlled by self-amplification of microtubules, which in turn is regulated by a gradient of active nucleators emanating from the DNA. This provides a robust mechanism for a steady state structure to maintain its size despite the rapid turnover of its building blocks.
Reference
Autocatalytic microtubule nucleation determines the size and mass of Xenopus laevis egg extract spindles. Decker F, Oriola D, Dalton B, Brugués J. eLife 2018;7:e31149. doi: 10.7554/eLife.31149.


































