Membraneless organelles are dynamic compartments inside cells that contain a complex mix of proteins and RNA. The mechanism of assembly of these compartments relies on a concentration dependent physico-chemical principle namely liquid-liquid phase separation, which is driven by weak molecular interactions between low complexity regions within proteins and RNA. Interestingly, it has been observed that during early mitosis, concomitant with nuclear envelope breakdown, multiple MLOs undergo dissolution resulting in complete redistribution of their constituents throughout the cell. However, towards the end of mitosis, these compartments start to condense back with the re-appearance of the nuclear envelope (see figure). The disassembly of these compartments during mitosis seems to be somewhat counter-intuitive. During mitosis the nuclear and cytoplasmic contents mix, which might result in the formation of aberrant hybrid MLOs that could be toxic to the cells. However, this does not occur, and the mechanism that prevents aberrant phase separation of proteins and RNA in mitotic cells is unexplored.
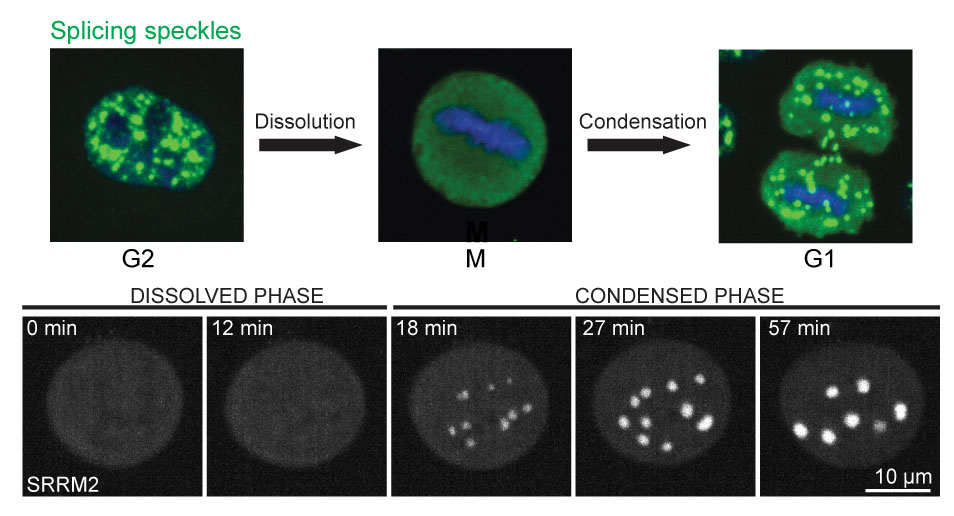
Figure: Top: Splicing speckle dynamics during cell cycle. Bottom: Time-lapse images of phase transition of a splicing speckle protein (SRRM2) from dissolved to condensed phase in mitotic cells upon DYRK3 inhibition.
In this study we show that small molecule inhibition of a dual-specificity kinase of the CMGC family, DYRK3, prevents dissolution of several compartments during mitosis, resulting in the formation of “hybrid” compartments in mitotic cells (see figure). These hybrid compartments constitute proteins from several compartments like stress granule, splicing speckle, pericentriolar satellite and spindle matrix. Multiple lines of evidence suggest that DYRK3 could possibly phosphorylate multiple resident proteins of MLOs, thus regulating their solubility during mitosis and in effect the condensed/dissolved state of the compartments. Further, we observed that owing to dilution of nuclear and cytoplasmic proteins upon nuclear envelope breakdown, the DYRK3 to substrate protein ratio increases by ~ 2.5 fold, which is sufficient for phase transition of a splicing speckle component from a condensed to a dissolved phase. This explains how DYRK3 could dissolve multiple MLOs during mitosis. The condensation during late mitosis can be explained by our observation that the APC/C driven ubiquitination degrades DYRK3 during M to G1 transition. The degradation of DYRK3 results in the lowering of DYRK3 to substrate ratio in early G1 that aids the cell to transit from a dissolved state back to a condensed state. Finally, the study shows that the inability to dissolve MLOs during mitosis delays mitotic progression and is toxic to the cells. This implies that dissolution of MLOs during mitosis is absolutely essential for cell survival.
Liquid-liquid phase separation has been shown to be fundamental to multiple aspects of cell biology and when un-regulated, phase separation could lead to disease conditions as observed in several neurodegenerative disorders. This work shows that in future, identifying additional proteins that regulate phase separation would provide us with the tools to prevent such processes from going awry inside cells.
Reference
Kinase-controlled phase transition of membraneless organelles in mitosis. Arpan Kumar Rai, Jia-Xuan Chen, Matthias Selbach, and Lucas Pelkmans. Nature. July 4, 2018. DOI: 10.1038/s41586-018-0279-8.


































