Muscle cells (myofibers) are designed to fulfill their purpose: to contract. Resulting from the fusion of hundreds of cells during development, one muscle cell grows into a large, multinucleated fiber. The cytoplasm of muscle cells is almost entirely occupied by sarcomeres, contractile units composed of overlapping actomyosin complexes and structural proteins. This unique architecture allows us to move around, whether to brush our teeth or run up stairs. But such mechanical pressure can lead to muscle damage, especially when stress is at its highest such as during exercise. Thankfully, the muscle organ possesses stem cells that activate to regenerate muscle cells after injury. These stem cells have astounding regenerative capabilities and have fascinated muscle biologists for decades. What we wondered in this study, was if muscle cells had the potential to repair injuries on their own, without stem cells.
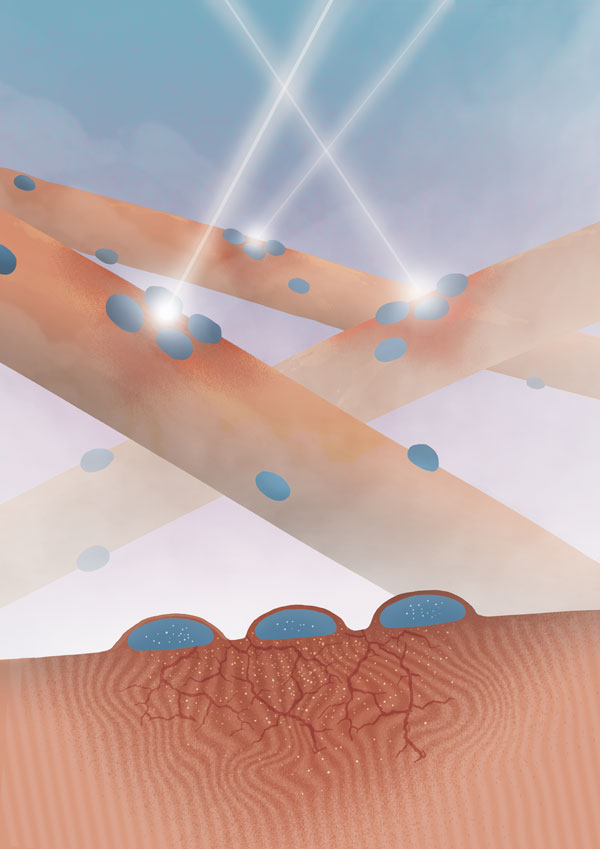
Figure: Myonuclei aggregate at an injury site where they locally deliver mRNA for cellular reconstruction. Image produce by Helena Pinheiro.
Using several models of local muscle damage, we identified a new mechanism of muscle regeneration: myofiber self-repair. Local damage to muscle cells triggers a signaling cascade involving calcium, Cdc42 and phosphokinase C that attracts nearby myonuclei to migrate to the injury site. The microtubule cytoskeleton and dynein motor proteins mediate these nuclear movements. Migrating myonuclei start expressing sarcomeric mRNAs, which are locally delivered at the injury site and translated into proteins for sarcomere reconstruction. This self-repair process occurs within 48 hours, is tailored to minor injuries and is independent of muscle stem cells.
Myofiber self-repair represents a new paradigm from which to understand muscle regeneration in exercise, disorders and aging. Acting as the first line of defense when facing muscle damage, myofiber self-repair is a promising cellular process that can be harnessed to preserve muscle integrity.
It is difficult to overstate the importance of the HFSP funding for the implementation of this project. The salary and research funds gave me the agency to explore the facets of the project I was most interested in. Moreover, the flexibility in the funding timeline and the assistance provided by the Fellowship Office were instrumental in tailoring my post-doc for my scientific development and the completion of the project.
This project opened more doors than it closed. I am currently seeking group leader positions in which I can explore further this new research line to tackle fundamental biological questions and translate these findings towards a societal impact.
|
HFSP award information Long-Term Fellowship (LT000133/2018-L): Discovering the mechanisms underlying intrinsic repair of muscle cells in wildtype and sarcopenia Fellow: William Roman |


































