Endogenously produced heat has been known for centuries as a factor contributing to body temperature, which is critical for the health of the entire organism. However, the small heat flux produced by a single isolated cell has been considered negligible and temperature has been thought to be an external parameter for such a cell.
Recent results in measurements of ultra-local temperature gradients inside living cells have prompted growing conceptual change in the understanding of intracellular heat management and the role of heat in cell functioning. The results have also triggered intense discussions in research literature because the highest increase of temperature within a micron-sized hot spot, which one can reasonably expect while adhering to the common physics law of heat dissipation, is more than 10,000 times smaller than has been previously observed and typically is only about 0.0001 of a degree Celsius. Apparently, a cell simply does not have enough energy to support a much higher temperature in the hot spot. It has been shown that the disagreement is not related to fundamental limitations in defining temperature for a nano-sized system because the fundamental limits related to the statistical nature of physical values in thermodynamics occur at a much smaller magnitude. Controversies related to the observed intracellular hot spots could not be resolved or explained without reliable and calibrated intracellular thermal measurements followed by a careful revision of the naïve approach based on common sense and conventional wisdom.
In a recent paper, HFSP Program Grant holders Dr Taras Plakhotnik and Dr Madoka Suzuki have demonstrated the first step along such an avenue by challenging one of the dominating beliefs postulating that thermal conductivity inside cells is close to that of water. It is not an unreasonable assumption since a cell has a lot of water inside. Their HFSP research project entitled “Nanoscale heat transfer phenomena: new paradigm for intra- and intercellular signalling and shaping” aims to solve the mystery of the hot spots.
The team members have co-authored a seminal paper published in Science Advances, which reports that the measured thermal intracellular conductivity turned out to be five to six times smaller than expected (the figure explains the idea of these measurements).
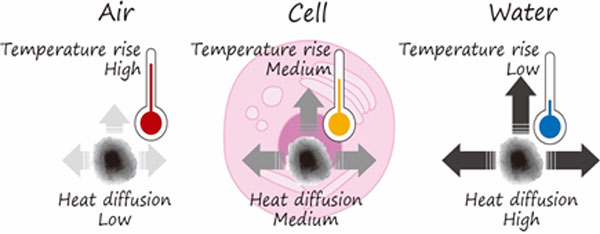
Figure: Schematics of heat conductivity measurements. A crystal of fluorescent nanodiamond (FND) of about 100 nm across is encapsulated in a 50-nm thick shell made of PDA, a polymer absorbing light and generating heat. The temperature of the diamond can be assessed optically and it depends on the thermal conductivity of the surrounding medium. The FND-PDA particles have been calibrated using known thermal conductivity values in water, air and oil and then used to measure thermal conductivity inside isolated cells.
The reduction of thermal conductivity is relatively small in comparison to the 10,000-fold excess above expectations in the temperature rise observed for the thermal hot spots and alone cannot explain the existence of the hotspots but the microscopic mechanism of the six-fold reduction also presents a mystery.
Understanding the process of heat diffusion inside cells is critical both fundamentally for understanding cell operation and for applications like cancer thermal therapy when temperature control is essential for the effectiveness and safety of the treatment. If the hot spots are real and the heat dissipation is reduced in comparison to the currently dominating beliefs, then heat generated in the hot spots can be used for intracellular signaling similar to the signaling based on diffusion of specific molecules to the targeted sites. The idea of thermal therapy relies on killing the cancer cells by raising their temperature. The healthy cells can tolerate a higher temperature but there is a limit to their tolerance. Better understanding of the heat diffusion process inside cells will be very beneficial for the effectiveness and safety of the treatment. Another example of practical significance is obesity. Accelerated cellular thermogenesis converting energy into heat is an effective approach to the treatment of obesity. However, it is essential to know how the released heat affects intracellular events such as biochemical reactions. Our approach will result in a tool which is capable of evaluating heat dissipation within a cell point-by-point. When the distribution of thermal properties in cells is understood, the way to dissipate heat more efficiently may be discovered. This will open the possibility of dissipating the energy gained from food efficiently without doing exhausting exercises.
Media coverage


































