Nucleus and DNA re-organize upon mechanical forces
It is particularly important to protect the genome of tissue-resident stem cells, which are long-lived, responsible for tissue maintenance, and are often subjected to mechanical forces. To study how the DNA in stem cells responds to mechanical deformation, we used a special mechanical device to expose skin epidermis and mesenchymal stem cells to similar mechanical stretch that they would experience inside the tissues. As a result of the stretch, both the nucleus and the DNA immediately re-organized and also changed their mechanical properties to become softer. If we experimentally prevented this change, the stem cells began to acquire DNA damage, indicating that we had discovered an important protective mechanism.
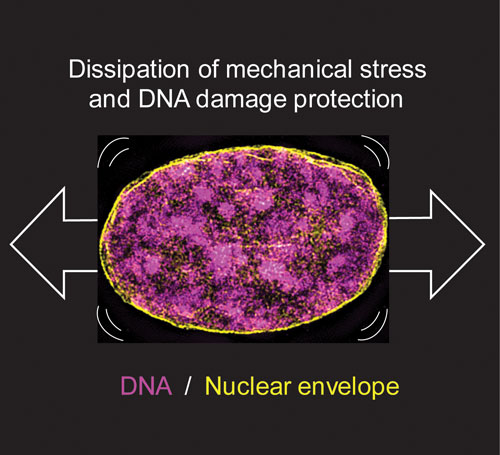
Figure: Super resolution microscopy image of a nucleus subjected to mechanical stretch. Stretch leads to changes in the physical properties of the nucleus and rearrangements in the 3-dimensional structure of the DNA, both of which are required for maintenance of DNA integrity under mechanical stretch.
Exposure to long-term mechanical stretch led to reorientation of the entire tissue in the direction of force. This tissue-scale orientation prevented further deformation of the nucleus and chromatin, allowing them to restore their original state. This tissue-level reorganization thus serves as a long-term mechanoprotection mechanism.
Cancer cells show impaired response to stretch
In contrast to healthy stem cells, cancer cells were less sensitive to mechanical stretch due to differences in the levels of key nuclear proteins that give the nucleus its structure and mechanical properties. Thus, we found that cancer cell nuclei are not only highly genetically instable and prone to acquisition of new mutations but also insensitive to extrinsic cues from their environments. Future studies will aim to understand the role of these newly discovered cancer cell nuclear features in the ability of cancer cells to exploit tissue mechanics to escape the control mechanisms of healthy tissue.
HFSP funding was instrumental in supporting the first co-author Dr. Yekaterina (Kate) Miroshnikova in this work now published in Cell. In particular, the funding enabled Kate to attend a number of international conferences, during which she initiated collaborations leading to key experimental findings, as well as facilitating visits to a number of collaborating labs for technology transfer. At the end of her HFSP Fellowship in 2021, Kate will return to the US to start her own laboratory at the National Institutes of Health (NIH) as a Stadtman Tenure-Track Investigator.


































