Frontotemporal dementia is the second most common type of dementia after Alzheimer’s disease and typically affects individuals below 65 years of age. In a subset of these patients, affected neurons present a characteristic pathology with cytoplasmic mislocalization and aggregation of the RNA-binding protein FUS, which normally resides in the nucleus. Since no mutations in FUS or any other proteins have been described in these cases, the trigger of FUS mislocalization that likely initiates the cascade of events leading to neuronal dysfunction and death remains enigmatic.
The new study by the group of HFSP Career Development Awardee Magdalini Polymenidou, shows that hypertonic stress leads to cytoplasmic translocation and loss of function of neuronal FUS, a protein that has the ability to phase separate and to incorporate into cytoplasmic stress granules. Surprisingly, and opposite to current thinking, the osmosis-triggered cytoplasmic shift of FUS is independent of stress granule formation or the molecular pathways induced by hyperosmolarity in cells.
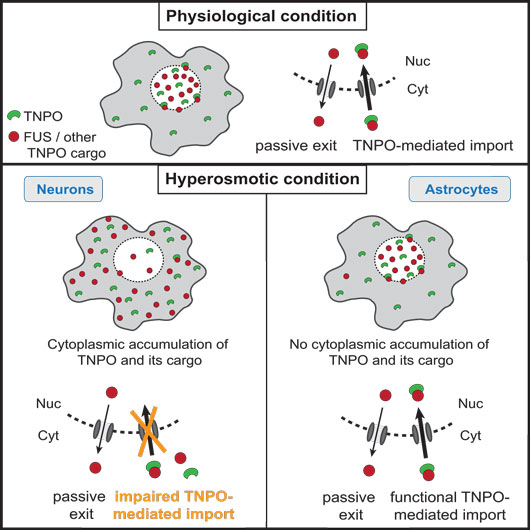
Figure first appeared in Cell Reports, 2018 Jul 24;24(4):987-1000.e7.
Instead, it is the Transportin pathway, which normally imports FUS from the cytoplasm into the nucleus, that is impaired under these conditions. Indeed, Transportin itself, as well as several of its protein cargo, which are also RNA-binding proteins related to FUS, are “stuck” in the cytoplasm during hyperosmolar shock. The authors hypothesize that this general blockade in Transportin function may represent a physiological protective response to exclude specific RNA-binding proteins from the nucleus, thereby inhibiting their function in RNA metabolism, while keeping them from aggregating.
Unexpectedly, astrocytes, glial cells that support neuronal function, are resistant to cytoplasmic FUS mislocalization upon hypertonic pressure, even though they are able to sense the hypertonic shock and to form stress granules. Using in vitro differentiated human neurons and astrocytes, the authors show that this resistance is an early event in the astrocytic fate commitment and occurs via a differential Transportin response in these cells. Importantly, astrocytes lack pathological protein accumulations in FTD-FUS brains, indicating a direct link between stress-induced FUS translocation and pathology.
An important implication of the work for public health is the fact that hyperosmolar therapy, which is the method of choice for release of inter-cranial pressure after brain trauma, may trigger the initial events that lead to FTD. Indeed, a strong association between brain trauma and FTD has been described in the past years by several groups, but no mechanism that could explain this association has been described to date. The Career Development Award from HFSP was instrumental for developing this project.
Reference
Hypertonic stress causes cytoplasmic translocation of neuronal but not astrocytic FUS due to impaired Transportin function. Hock EM, Maniecka Z, Hruska-Plochan M, Reber S, Laferriere F, Sahadevan, SMK, Ederle H, Gittings L, Pelkmans L, Dupuis L, Lashley T, Ruepp M, Dormann D, Polymenidou M. (2018) Cell Reports, 2018 Jul 24;24(4):987-1000.e7. doi: 10.1016/j.celrep.2018.06.094.


































