The bottom-up construction of synthetic cell systems is a thriving research area that addresses fundamental questions related to the origin of unicellular life and that challenges our current capabilities to engineer synthetic life forms from molecular components. One of the bottlenecks is achieving the physical growth of cell-sized compartments made of a phospholipid membrane boundary capable of encapsulating complex enzymatic reactions such as in vitro protein expression. An important question is how this type of protocell model could have physically grown without sophisticated enzymatic mechanisms such as phospholipid synthesis or cytoskeleton-based processes.
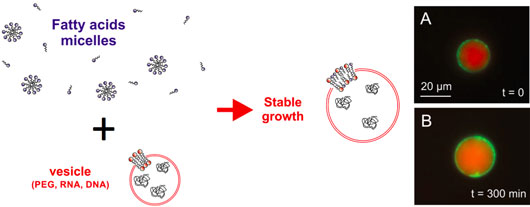
Figure: Growth of phospholipid vesicles in a bath of fatty acids. Vesicles loaded with a cell-free expression system and plasmid DNA are prepared and added to an aqueous solution containing fatty acids micelles. At specific fatty acid concentrations, the vesicles grow in a stable manner while protein expression of a reporter gene occurs, as observed on the right where a fluorescent reporter protein (green) is expressed and interacts with the membrane. With Kind Permission of the European Physical Journal (EJP).
In this work, the Libchaber and Noireaux labs demonstrate that cell-sized phospholipids vesicles, loaded with a cell-free expression system and plasmid DNA to express a reporter protein, can physically expand when they are added to a solution containing fatty acid micelles (Figure 1). Fatty acids, found in meteorites for example, are primitive membrane building blocks that form micelles in aqueous solutions. The researchers have determined that the four observed regimes (no growth, stable growth, unstable growth and bursting) depend on the concentration of fatty acids added to the external solution and on the osmotic pressure between the vesicles and the outer solution. They demonstrate that in a relatively large part of this parameter space the phospholipid vesicles grow in stable conditions by spontaneous insertion of fatty acids into the phospholipid membranes of the vesicles. Phospholipids and fatty acids can mix together in thin lipidic bilayer membranes because they are both amphiphilic molecules: they have a hydrophilic part and a hydrophobic part. During the growth process, in vitro protein expression inside the vesicles remains active as observed by fluorescence microscopy (Figure 1). Some of the liposomes bud, which could have served as a step towards division or fission. This work contributes to understanding how protocells could have achieved physical transformation by pure physical means.
The Libchaber and Noireaux labs provide one possible scenario for how primitive cells, capable of recapitulating a process as complex as gene expression, could have physically grown under conditions that do not require highly evolved enzymatic mechanisms for membrane synthesis. Developing other schemes that could explain non-enzymatic self-reproduction of primitive cells will require more investigation.
Reference
Growth and instability of a phospholipid vesicle in a bath of fatty acids. Dervaux, J., Noireaux, V., Libchaber, A. Eur. Phys. J. Plus (2017) 132: 284. DOI 10.1140/epjp/i2017-11554-1.


































