Sexually reproducing animals display sex-specific behaviors. Males and females respond to environmental sensory cues and transform the input into dimorphic behaviors. While sexual dimorphisms in brain structure and function are evident across phylogeny, sexually dimorphic features of neurons are not well defined. Understanding the mechanisms that generate sexually dimorphic wiring patterns could prove beneficial in the development of novel gender-specific therapies.
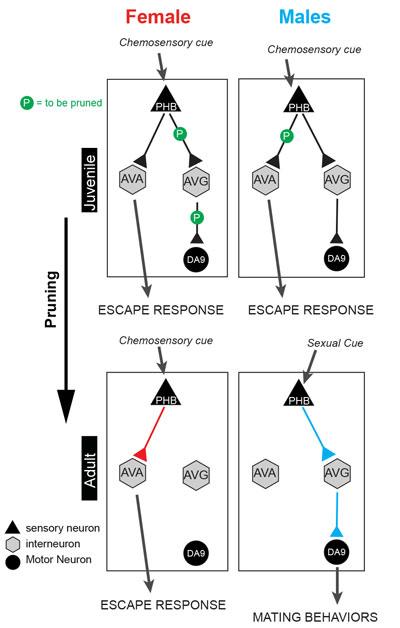
Figure: Sexually dimorphic connectivity patterns arise during development by pruning of synaptic connections. The PHB sensory neurons function as chemosensory cells that negatively modulate reversals to odorant repellents. In hermaphrodites, PHBs synapse onto command interneurons (AVAs) that control locomotion. Male PHBs do not process the same sensory cues as in hermaphrodites, but instead are repurposed to process cues involved in mating behavior. PHB-AVA synaptic connections are shared by both sexes at the L1 stage, therefore resulting in the sensory modulation of locomotion by repellents in both sexes. During development, the PHB's synapses to AVA interneurons are pruned in males, generating a sexually diverged synaptic state that generates dimorphic behaviours.
In the nematode C. elegans, like other invertebrate or vertebrate systems, the vast majority of the neurons are shared between the sexes, but a small number of neurons are found exclusively in male or female brains. The sex-shared neurons are strongly sexually dimorphic in their synaptic connectivity patterns.
We found that in the worm's juvenile state, neuronal connections were in a hybrid state, comprised of both male and female arrangements. Upon sexual maturation neuronal connections underwent a pruning process, eliminating particular connections and generating either male or female patterns. This is the first demonstration in any system that sex-specificity arises by pruning of synapses.
We undertook behavioral tests to define sex-specific behaviors mediated by the dimorphically connected neurons and showed that the rewiring is indicative of repurposing of the function of sensory and interneurons. The female and male versions of the same sensory neuron (PHB) work differently in the two sexes. In hermaphrodites it functions to avoid a noxious chemical cue, but in males this neuron is differentially connected and functions in recognizing mating cues. However, early in development, the PHB neuron in males also responded to signals regulating taste, suggesting that even though those neurons are found in both sexes, in adults sex-specific wiring results in repurposing of their function. By using neuron-type specific conversions of sexual identity we show that the sex of the presynaptic neuron is a determinant of sex-specific wiring patterns (i.e. to decide whether to keep or prune a synapse). At the genetic level, we have linked the globally-acting sex determination system and synaptic pruning, and showed that two phylogenetically conserved transcription factors of the Doublesex family (DM domain Zn finger transcription factors, involved in sex-determination in worms, flies and mice) are dimorphically expressed in the dimorphically connected neurons. Moreover, we showed that they are not only required but, strikingly, also sufficient to determine sex-specific wiring.
Reference
Sex-specific pruning of neuronal synapses in Caenorhabditis elegans. Oren-Suissa M., Bayer EA and Hobert O. Nature. 2016 May 4;533(7602):206-11. doi: 10.1038/nature17977. PMID: 27144354.
Nature News and Views commentary


































