Benjamin Palmer, an HFSP Cross-Disciplinary Fellow, describes his research in the emerging field of organic biomineralization, and in particular on the intricate structure and functioning of the mirrored eye of the scallop. Dr Palmer works in the lab of HFSP Program Grant holder Lia Addadi and Steve Weiner at the Weizmann Institute of Science in Israel.
Most people are surprised to hear that scallops see with up to 200 majestic iridescent eyes peering out from between their two valves (Fig. 1A,B). We too were ignorant of this hidden treasure until reading the work of Professor Michael Land while preparing a review article on natural photonics in 2016 [1]. Land performed pioneering research on vision from the 1960s to the 80s, including uncovering the optical secrets of the mirrored eyes of animals.
Most terrestrial animals, including humans, use lenses to form images by refracting light at an interface between two media with different refractive indexes. Conventional lens-based eyes work well in terrestrial environments, but things start to get more complicated under water, where the contrast in the refractive index between the outside medium (water) and the cornea is much smaller. This limitation, together with other selective pressures associated with seeing under water (e.g. low light intensities and the preferential attenuation of red light by water), has resulted in numerous ‘ingenious’ solutions to the problem of vision in aquatic animals. One of these solutions is to use mirrors instead of lenses to form images.
A spectacular example of a mirrored eye is in the scallop. Land found that scallops form images by reflecting light from a concave mirror lining the back of their eyes onto a double-layered retina residing above it [2]. He determined that this mirror was made from guanine crystals which are responsible for its beautiful iridescent appearance.
Despite their beauty, scallop eyes remained a curiosity for us until Ben Palmer had a serendipitous conversation with a local fisherman whilst holidaying at home in South Wales. The fisherman reported observing thousands of ‘diamonds’ shining out of the darkness when diving under torchlight over a scallop bed off the Pembrokeshire coast... and so having inadvertently been presented with a source for our scallops, our journey with their eyes began…
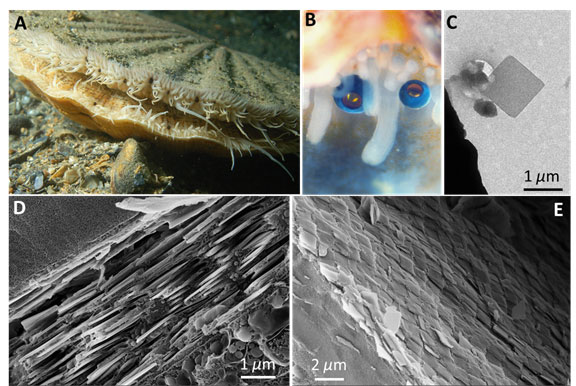
Figure 1. (A) The scallop Pecten maximus with numerous eyes (appearing black in this image) dotted along the mantle tissue (photograph courtesy of Mr. Ceri Jones of Haven Diving Services). (B) A higher magnification view of two scallop eyes (photograph courtesy of Prof. Dan-Eric Nilsson of Lund University). (C) A TEM of a single square guanine crystal extracted from the scallop eye mirror. (D) and (E) Cryo-SEM images of the mirror viewed (D) perpendicular to the mirror showing the multilayer structure and viewed (E) above the mirror showing the crystal tiling.
Our research focuses on the way in which animals use crystalline materials to manipulate light, for example, to produce structural colors used in camouflage and display [1, 3]. However, we had never looked at eyes before and we were intrigued to understand how an image-forming mirror is constructed from biological materials and what its optical performance is.
One novel aspect of our research was to use cryogenic scanning electron microscopy to observe the nano to microscale organization of the scallop eye mirror in close-to-life conditions [4]. The scallop mirror is constructed from millions of micron-sized square guanine crystals (Fig. 1C) which are arranged into a multilayer formed from 20-30 layers of crystals separated by cytoplasm (Fig. 1D). This is an example of a multilayer interference mirror whereby light is reflected from the interfaces between the high refractive index guanine crystals and the low refractive index cytoplasm. We also obtained high resolution 3D images of the fresh hydrated eye using X-ray microCT. This too enabled us to resolve the detailed anatomy under almost in vivo conditions.
The square-plate morphology of the crystals is extremely unusual, being observed nowhere else in the animal kingdom, and is a clear indication of the control which the organism exerts over the crystallization process. Each square ‘single crystal’ is in fact constructed from a sandwich of smaller sub-crystals called crystal-twins. Control over the orientational relationship between these twin components is used as a strategy for controlling the crystal morphology [5].
The entire surface of the scallop’s mirror is tessellated with an ordered mosaic of square crystal tiles – closely resembling the segmented mirror of reflecting telescopes (Fig. 1E). Crystal tiling appears to be functionally important, since tiling the crystals together minimizes defects in the mirrors surface, which in turn minimizes optical diffraction aberrations and optical loss due to transmission of light through the mirror.
Other properties of the multilayer mirror also appear to be well-suited to the visual needs of the scallop. Reflectivity simulations demonstrated that the crystal thicknesses and cytoplasm spacings of the mirror are ‘optimized’ to efficiently reflect the blue green light that penetrates the scallop marine habitat.
At a higher level of organization the scallop mirror has a concave shape. X-ray micro computed tomography showed that it does not have a simple hemispherical shape as was previously thought. Rather, the central portion of the mirror is flattened with respect to the periphery. Optical ray tracing simulations indicated that this has an important consequence for the visual performance of the eye. The unusual shape of the mirror means that it has two focal points at different heights above the surface depending on the angle of incidence of the light. This may be the key to understanding why the scallop has an unusual double-tiered retina.
Light impinging on the eye from on-axis sources is focused predominantly on the upper (distal retina), whereas light hitting the periphery of the mirror is focused predominantly on the lower (proximal) retina. Previous studies had shown that the distal retina responds to dark, moving features, triggering defense or escape reflexes but the role of the underlying proximal retina was not known. Ray tracing showed that dim, peripheral light forms well resolved images on the highly sensitive proximal retina, which we suggest may be specialized to observe static features of its habitat in poorly illuminated conditions.
Scallop eyes provide another example of the extraordinary control which organisms exert over both the morphology and organization of crystals to produce different optical phenomena. One of the key goals in this emerging field of organic biomineralization is to understand how organisms control crystallization and to determine if these strategies could be harnessed in the development of new materials, bearing in mind that many biological optical devices display functionalities beyond the state of the art in artificial optics.
We are also interested to explore what other functional organic crystals, besides guanine, exist in nature. In comparison to inorganic bio-minerals very few types of functional organic crystals are known to exist. Isoxanthopterin, a pteridine analogue of guanine, was recently found to be the reflective material in the mirrored eyes of decapod crustaceans, and it may well be that many other types of functional organic crystals remain to be discovered [6]. Exploring the fascinating biological functions that these organic biogenic crystals perform will no doubt provide fruitful ground for future research.
|
References [1] D. Gur, B.A. Palmer, S. Weiner, L. Addadi, Adv. Func. Mater. 2017, 1603514. [2] M. F. Land, J. Physiol. 1965, 179, 138-153. [3] (a) D. Gur, B.A. Palmer, B. Leshem, D. Oron, P. Fratzl, S. Weiner, L. Addadi, Angew. Chem. Int. Edit. 2015, 54, 12426-12430; [4] B.A. Palmer, G.J. Taylor, V. Brumfeld, D. Gur, M. Shemesh, N. Elad, A. Osherov, D. Oron, S. Weiner, L. Addadi, Science 2017, 358, 1172-1175. [5] A. Hirsch, B.A. Palmer, N. Elad, D. Gur, S. Weiner, L. Addadi, L. Kronik, L. Leiserowitz, Angew. Chem. Int. Ed. 2017, 56, 9420-9424. [6] B.A. Palmer, A. Hirsch, V. Brumfeld, E. D. Aflalo, I. Pinkas, A. Sagi, S. Rozenne, D. Oron, L. Leiserowitz, L. Kronik, S. Weiner, L. Addadi, accepted PNAS 2018. |
 Benjamin Palmer is an HFSP Fellow at the Weizmann Institute of Science in Israel. He studied chemistry in his home city of Cardiff and continued there for his PhD in solid state organic chemistry under the supervision of Prof. Kenneth Harris. His PhD research on the interaction of polarized X-rays with materials led to the development of the X-ray Birefringence Imaging (XBI) technique. In 2014, he joined the group of Profs. Lia Addadi and Steve Weiner at Weizmann as a Dean of Faculty and Koshland Prize Fellow. Since 2015, he has been funded by an HFSP Cross-Disciplinary Fellowship. His research is in the field of ‘organic biomineralization’ and he is particularly interested in how organisms make and use organic crystals to manipulate light for different optical functions. Ben has a special interest in visual systems in animals which utilize reflective rather than refractive optics. Benjamin Palmer is an HFSP Fellow at the Weizmann Institute of Science in Israel. He studied chemistry in his home city of Cardiff and continued there for his PhD in solid state organic chemistry under the supervision of Prof. Kenneth Harris. His PhD research on the interaction of polarized X-rays with materials led to the development of the X-ray Birefringence Imaging (XBI) technique. In 2014, he joined the group of Profs. Lia Addadi and Steve Weiner at Weizmann as a Dean of Faculty and Koshland Prize Fellow. Since 2015, he has been funded by an HFSP Cross-Disciplinary Fellowship. His research is in the field of ‘organic biomineralization’ and he is particularly interested in how organisms make and use organic crystals to manipulate light for different optical functions. Ben has a special interest in visual systems in animals which utilize reflective rather than refractive optics. |
 Lia Addadi is a Professor at the Weizmann Institute of Science in Israel. Her research (much of which is conducted in collaboration with Prof. Steve Weiner) spans the mechanisms of biomineralization, cell adhesion, and antibody recognition of organized materials, and has implications in areas as diverse as materials research, optics and medicine. Of particular interest is currently the research on how organisms manipulate light through complex crystalline architectures in plant leaves, fish scales, crustacean cuticles and invertebrate eyes. Lia currently holds an HFSP Program Grant to investigate the role of extracellular vesicles in breast cancer bone metastasis. Addadi’s work has been recognized with the award of the Prelog Medal in Stereochemistry (1998), the 2009 Prize for Excellence from the Israel Chemical Society, the 2011 Aminoff Prize for Crystallography by the Swedish Royal Academy of Sciences, and in 2017 with her election to the US Academy of Sciences. Lia Addadi is a Professor at the Weizmann Institute of Science in Israel. Her research (much of which is conducted in collaboration with Prof. Steve Weiner) spans the mechanisms of biomineralization, cell adhesion, and antibody recognition of organized materials, and has implications in areas as diverse as materials research, optics and medicine. Of particular interest is currently the research on how organisms manipulate light through complex crystalline architectures in plant leaves, fish scales, crustacean cuticles and invertebrate eyes. Lia currently holds an HFSP Program Grant to investigate the role of extracellular vesicles in breast cancer bone metastasis. Addadi’s work has been recognized with the award of the Prelog Medal in Stereochemistry (1998), the 2009 Prize for Excellence from the Israel Chemical Society, the 2011 Aminoff Prize for Crystallography by the Swedish Royal Academy of Sciences, and in 2017 with her election to the US Academy of Sciences. |
 Born in Pretoria, South Africa, in 1948, Steve Weiner attained a BSc in geology and chemistry from the University of Cape Town in 1969; that same year, he made aliyah to Israel. He went on to receive a MSc in geochemistry from the Hebrew University of Jerusalem and a PhD in biomineralization from the California Institute of Technology. He joined the Weizmann Institute in 1977. Prof. Weiner carries out research in two fields: biomineralization, the processes by which organisms produce minerals, and microarchaeology, revealing the microscopic archaeological record with the help of instruments. Prof. Weiner received the Israel Chemistry Society Prize for Scientific Excellence in 2009 and the Aminoff Prize for Crystallography from the Royal Swedish Academy of Sciences in 2011. In 2013, he received the Pomerance Award for Scientific Contributions to Archaeology. Together with the late Prof. Heinz Lowenstam, Prof. Weiner wrote the book On Biomineralization (Oxford University Press, 1989), a widely used text book in this field, and the book Microarchaeology: Beyond the Visible Archaeological Record (Cambridge University Press, 2010). Born in Pretoria, South Africa, in 1948, Steve Weiner attained a BSc in geology and chemistry from the University of Cape Town in 1969; that same year, he made aliyah to Israel. He went on to receive a MSc in geochemistry from the Hebrew University of Jerusalem and a PhD in biomineralization from the California Institute of Technology. He joined the Weizmann Institute in 1977. Prof. Weiner carries out research in two fields: biomineralization, the processes by which organisms produce minerals, and microarchaeology, revealing the microscopic archaeological record with the help of instruments. Prof. Weiner received the Israel Chemistry Society Prize for Scientific Excellence in 2009 and the Aminoff Prize for Crystallography from the Royal Swedish Academy of Sciences in 2011. In 2013, he received the Pomerance Award for Scientific Contributions to Archaeology. Together with the late Prof. Heinz Lowenstam, Prof. Weiner wrote the book On Biomineralization (Oxford University Press, 1989), a widely used text book in this field, and the book Microarchaeology: Beyond the Visible Archaeological Record (Cambridge University Press, 2010). |


































