An accepted uniting character of all living sharks, rays, and chimaera (the so-called cartilaginous fishes) is the presence of a mineralized crust on the outside of the skeleton, broken into many thousands of minute plates called tesserae. Tesserae are thought to be one of the keys to the evolutionary success of sharks and rays, yet had never been demonstrated in modern chimaera and it was debated whether chimaera even had the capacity for cartilage mineralization. In our recent study in the Journal of the Royal Society Interface, we finally show that the chimaera lineage did not do away with tesserae during evolution and that the tessellated cartilage of modern chimaera is in many ways similar to that of sharks and rays, albeit with a series of curious differences.
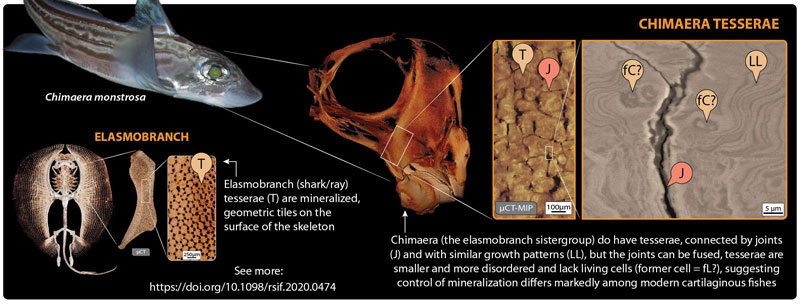
Tesserae in sharks and rays are mostly quite small, just barely visible to the naked eye. However, their impressive material properties (more similar to teeth than bone), their sheer abundance, and their complex arrangements —all aspects explored and quantified in our HFSP work— are believed to create a mechanical ‘exoskeleton’ that allows shark and ray cartilage to out-perform mammal cartilage in many ways. A key to understanding how this composite biological material works is to understand its variation in relation to its function (e.g., which species have which types of tesserae and how tesserae diversity relates to species’ ecology). Curiously though, for decades researchers could not agree whether chimaera (the furtive and less-lauded sister-group of sharks and rays) even had tesserae at all. In our JRS Interface paper, we settle this debate, applying to the study of chimaera skeletal tissue the same high-resolution and 3D imaging and characterization tools we had used to help fuel the upsurge in shark/ray cartilage research in the past decade.
In our paper, we show that not only is tessellated cartilage indeed present in chimaera, but that it shares multiple specific features with that of sharks and rays. For example, chimaera tesserae, like shark/ray tesserae, are linked by flexible joints, with contact points reinforced by repeating structural patterns of extremely high mineral content. This argues that many of the tiniest, but mechanically important structural details of tesserae were hit upon hundreds of millions of years ago (before the split between the chimaera and shark/ray lineages) and then held on to by both groups. Oddly though, chimaera tesserae appear to lack the striking internal cell networks of shark/ray tesserae, suggesting fundamental differences between groups in how their cartilage cells behave (e.g., how calcification is controlled).
We could only offer the proper context to our chimaera studies by diving back into the shark/ray cartilage anatomy and development data we generated earlier during our HFSP funding. In this way, our JRS Interface article, comparing tessellated cartilage in all groups of living cartilaginous fishes, ended up a perfect capstone for the diverse biological data made possible by our grant. Synthesizing our grant’s insights —from the morphology of skeletons down to their tissues and materials— we framed the state of the art for the study of an emerging model system (e.g., for cartilage biology research), while opening questions for future work. Since tesserae are one of the few tissues to fossilize in extinct cartilaginous fishes, our findings also offer a deep window into the evolution and true diversity of cartilage and bone in vertebrates, and the steps taken by Nature to craft distinctive biological materials.
Text by Ronald Seidel and Mason Dean


































