Centrosomes have many important cellular functions. One among them is to serve as the major microtubule-organizing center (MTOC) in regulating the organization of bi-polar spindles for accurate cell division. At their core, centrosomes are composed of a pair of centrioles surrounded by peri-centriolar material (PCM) formed by various multi-protein complexes. At interphase of the cell cycle where centrioles duplicate, the centrosome contains a basal level of PCM and is incompetent to nucleate robust microtubules. However, as cells enter into mitosis, duplicated centrosomes recruit PCM and expand in size. The process of PCM recruitment enabling centrosomes to be functional organelle in mitosis is termed as centrosome maturation. Via our interdisciplinary collaboration supported by HFSP, we recently identified that Sas-4 (in flies) / CPAP (in human), a conserved centrosomal protein, plays a role in centrosome maturation (the laboratories of Jay Gopalakrishnan and Haitao Li. Zheng et al. Nature Communications 2016).
How centrosomes mature, in particular the mechanisms that regulate PCM recruitment to control centrosome size and capability, has remained unclear. In one of our studies, by generating cell cycle specific Sas-4 antibodies, we identified that in Drosophila, Sas-4 can be phosphorylated by Plk1/Polo kinase (Ref [1]). This phosphorylation appears to occur at the onset of mitosis, enabling Sas-4 to expand its localization, growing outward from meiotic and mitotic centrosomes. Thus in this work, we identify a previously unknown role for Plk1/Polo kinase that spatiotemporally activates Sas-4 to determine centrosome size and build functional centrosomes.
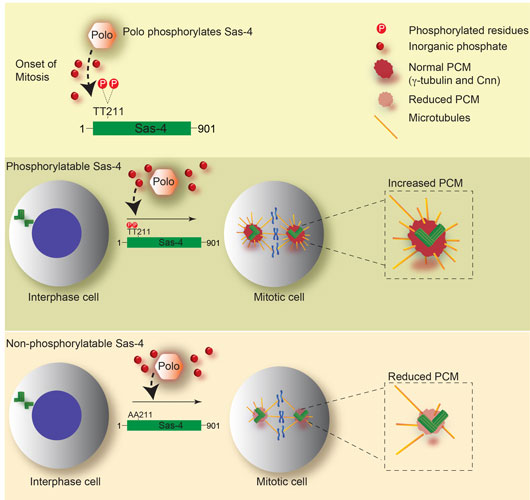
Figure 1: Mechanism of building mitotic centrosomes
In a separate study (Ref [2]), we targeted the centrosome activation mechanisms in cancer cells. Centrosome duplication is restricted to only once per cycle. In contrast to normal cells, cancer cells harbor extra centrosomes and thus centrosome amplification is a hallmark of human cancers that can trigger cancer cell invasion. Amplified extra centrosomes should in theory cause multipolar mitosis, leading cancer cells to undergo mitotic catastrophe and cell death. However, cells with extra centrosomes achieve a pseudo-bipolar spindle via centrosome clustering, a key mechanism by which cancer cells cluster their extra centrosomes to circumvent mitotic catastrophe. When cells fail to cluster extra centrosomes in mitosis, they have been shown to undergo multipolar divisions and enter apoptosis. Thus, inhibiting centrosome clustering to induce multipolar divisions has been proposed as a strategy to counteract tumors with high incidences of centrosome amplification. How extra centrosomes can be manipulated to prevent them from clustering remained largely unknown.
Tubulin, by interacting with the centrosomal protein CPAP, negatively regulates CPAP-dependent PCM recruitment and concurrently microtubule nucleation. We screened for compounds that perturb CPAP-Tubulin interaction leading to the identification of CCB02, which selectively binds at the CPAP binding site of tubulin. Genetic and chemical perturbation of CPAP-Tubulin interaction activates extra centrosomes to nucleate enhanced levels of microtubules prior to mitosis. This caused cells to undergo centrosome declustering, prolonged multipolar mitosis and cell death. CCB02 but not conventional tubulin binders activated extra centrosomes to nucleate an enhanced level of microtubules prior to mitosis and prevented them from clustering. 3D-organotypic invasive assays revealed that CCB02 has a broad anti-invasive activity against various cancers including tyrosine-kinase inhibitor (TKI)-resistant EGFR-mutant non-small cell lung cancers. Thus, we have identified a vulnerability of cancer cells to extra centrosomal activation, which may serve as a global approach to target various cancers including drug-resistant cancers exhibiting a high incidence of centrosome amplification. The CPAP-Tubulin inhibitor CCB02 may not only serve as a useful tool to study centrosome functions in cells, but also as a starting point for developing combinatorial treatment strategies, specifically when extra centrosomes indirectly contribute to a “bypass track” by which therapy-resistant cancers develop.
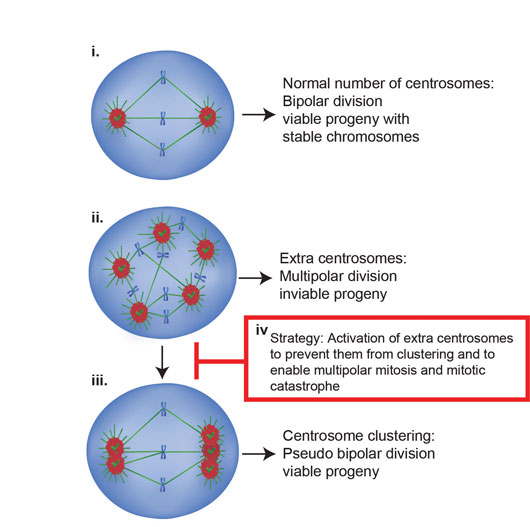
Figure 2: Mechanism to selectively eliminate cells with extra centrosomes


































