The inside of a human cell is divided into compartments called organelles, which are surrounded by membranes. Each organelle plays a specific role in keeping the cell healthy and also has a unique mix of molecular markers on its surface. These markers allow other molecules to identify the different organelles; specific organelles can communicate with each other and coordinate their activities. However, how exactly is information relayed between organelles? To tackle this question, FoSheng Hsu, a HFSP Long-Term Fellow in the Zerial lab, embarked on an exploration delving into inter-organelle communication, and surreptitiously, discovered a physical connection between mitochondria and endosomes occurring when cells experience high levels of oxidative stress – a condition by which the levels of toxic chemicals called reactive oxygen species become too high.
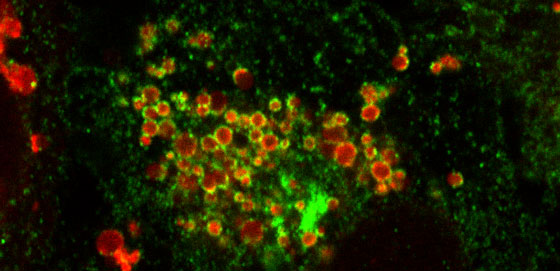
Figure: Inside of a human cell during oxidative stress; the mitochondria are labeled by a chemical dye (red) and the protein Rab5 is expressing a green fluorescent tag (green). The image shows Rab5 forming a ring-like structure around mitochondria.
Mitochondria are organelles that not only provide energy inside human cells, but also work to keep the levels of oxidative stress in check. The surprising finding that endosomes, which are responsible for sorting materials within the cell, can physically interact with mitochondria led an international team (Hyman & Zerial laboratories at MPI-CBG and Parton laboratory at University of Queensland, Australia) to investigate the physiological relevance of this phenomenon.
In many neurodegenerative diseases, the mitochondria and endosomes in neurons do not work properly. Interestingly, these cells also have higher than normal levels of oxidative stress. Using human stem cells grown in culture and inducing them to become neurons, the team discovered a gene called ALS2/Alsin, which is mutated in early-onset amyotrophic lateral sclerosis (ALS), is targeted to the surface of mitochondria upon oxidative stress. Furthermore, ALS2/Alsin functions as a vehicle to bring a protein called Rab5, usually found on endosomes, to mitochondria. This stress response turns out to be a critical determinant of life or death for the neurons.
Overall, this study shed light on the pathogenetic mechanism for ALS and opens a new front to further explore whether defects in endosomes or mitochondria, and specifically with molecules like ALS2/Alsin and Rab5, are responsible for other neurodegenerative disorders like Parkinson’s disease and Huntington’s disease.
Reference
Rab5 and Alsin regulate stress-activated cytoprotective signaling on mitochondria. Hsu F, Spannl S, Ferguson C, Hyman AA, Parton RG, Zerial M. eLife (2018). doi: 10.7554/eLife.32282.


































