In a recent study we have introduced a biomimetic system for reversibly driving intracellular phase separation in a spatiotemporally controlled manner using photo-induced oligomerization of weakly interacting proteins. We utilized the quantitative nature of this platform to map, for the first time, full intracellular phase diagrams, which deterministically dictate whether and how phase separation occurs and quantitatively describes how specific mutations and post-translational modification modulate the transition. Moreover, we show that while intracellular protein concentration may be insufficient for global phase separation, locally tuning the conjugation number of phase-separating proteins to slowly diffuse scaffolds can move the cell into a different region of the phase diagram, resulting in localized phase separation. We suggest that this diffusive capture mechanism liberates the cell from the constraints of global protein abundance and is likely exploited to pattern condensates associated with diverse biological processes.
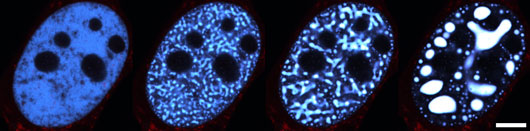
Figure: Nucleus of a representative U2OS type cell (human bone cancer) undergoing phase separation. The transition from a uniform single-phase state (left) into a two-phase state, in which condensates of concentrated proteins (white blobs) coexist with their dilute surrounding, is initiated by blue light activation and lasts for two minutes. Scale bar is 10 µm.


































