Using a bottom-up approach to combine magnetic nanoparticles, synthetic RNAs, and human cell extracts, all confined in a water-in-oil droplet, we observed that a single messenger RNA not only can be translated into functional proteins, but can also serve as a spatiotemporal scaffold to concentrate the nascent proteins and their function at the translation site over the time of translation. This temporary site-specific concentration is observed to trigger structural polymorphism of actin filament mesh works, the choreographers of cell shape and function.
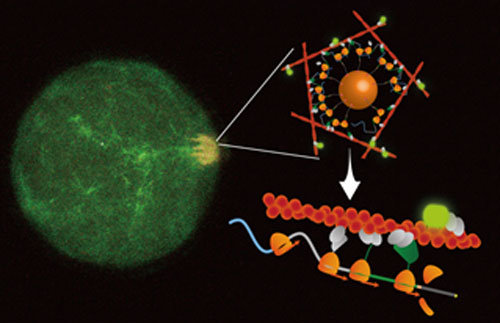
Figure: Confocal observation showing the formation of actin filaments (orange patch) triggered by the translation of mRNA molecules grafted on magnetic nanoparticles (depicted in the schematic). Being able to confine locally mRNA translation using magnetic nanoparticles helped to unravel the role of mRNA as a translation-coupled scaffold with an anchoring function. Credit: Shunnichi Kashida and Zoher Gueroui.
The spatial regulation of messenger RNA (mRNA) translation significantly contributes to many important cellular processes, such as the establishment of polarity, asymmetric division, synaptic plasticity, and memory consolidation. Many studies have increased our understanding of the molecules and reaction mechanisms involved in the mRNA localization and local translation, but none of these approaches succeeds in unravelling the causality between local translation RNA processing and cell functions. Understanding the spatiotemporal nature of translation is challenging and requires the development of novel orthogonal methods for harnessing biomolecule functions.
Here, the researchers introduced a novel approach based on the use of tiny nanoparticles that conjugate to mRNA molecules, enabling the manipulation of biomolecules in space using magnetic forces. When confined into micrometer-size droplets of human cell extracts, mRNA-nanoparticle conjugates act as movable mRNA-based protein factories upon experiencing a magnetic field. This new technology allowed the researchers to bypass subcellular transport systems and to uncouple endogenous biochemical regulation from physical constraints. The mRNA-nanoparticle complexes operate as a source for the continuous production of proteins from defined positions. This approach highlights a novel mechanism showing how functional protein domains can be spatially retained on mRNA-nanoparticles via ribosomes during translation. The results reveal an unexpected role of mRNA as an anchoring molecule to create a local concentration and function of nascent protein domains during translation. To illustrate its potential functional relevance to cell functions, the researchers examined the spatial localization of mRNAs coding for actin cytoskeleton-related proteins, which are Important for various processes such as cell polarization and migration. By applying a magnetic field to localize mRNAs coding for actin-binding proteins, they demonstrated the triggering of actin cytoskeleton formation and identified the minimal ingredients required for spatial control.
The next challenge is to find whether and/or how this mechanism happens in living cells. The results could be very interesting, because they may unravel how cells use the same molecular toolkit to achieve large functional diversity.
The authors acknowledge HFSP support to help getting this project off the ground. "I thought I understood local translation in neurons after years of imaging and molecular analyses, but this HFSP-funded study with Zoher and Hiro using a multi-disciplinary, bottom-up approach, has offered us an opportunity to come to a whole new concept. Retrospectively, this is such a simple, clever and economical mechanism that cells may engage to generate functional diversity of proteins through mRNA. But we had no idea three years ago. This is the frontier and it has a great view from here!", says Ohtan.


































