Fertilization of sperm and an egg triggers rapid and drastic changes, in particular, in their nuclear organelles, promoting the swelling and growth of nuclei, referred to as pronuclei. In pronuclei, a series of reprogramming events occurs in order to obtain developmental abilities of fertilized embryos. These include the decompaction of chromatin and chemical modifications on DNA and its associated histone proteins, which enables embryos to set up proper gene expression programs. Meantime, such dramatic changes make embryonic chromatin susceptible to DNA damage that needs to be repaired for subsequent development. Therefore, the proper formation and functions of pronuclei with DNA repair activity are critical for early development. How functional pronuclei are formed and organized is poorly understood.
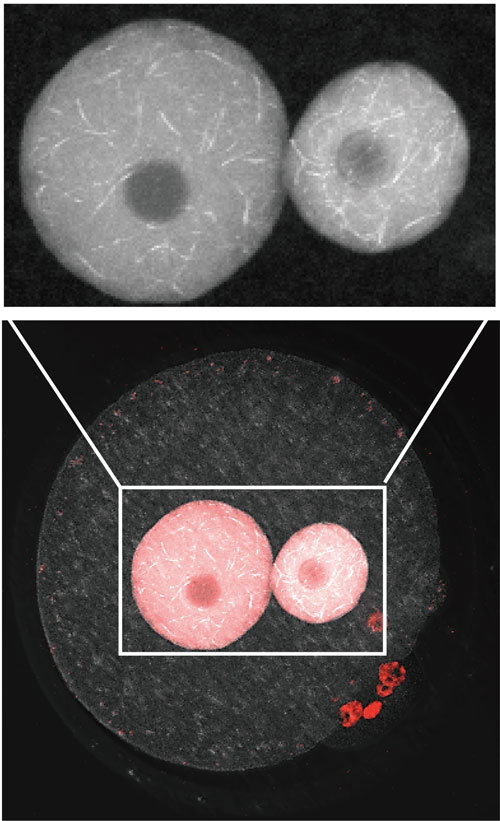
Figure: Nuclear actin filament formation in pronuclei of a mouse embryo at the one-cell stage. Nuclear actin filaments are visualized by an anti-actin chromobody (shown in white). Pronuclear areas are indicated by DNA staining (shown in red). The enlarged image shows male (left) and female (right) pronuclei.
In our HFSP collaboration we aimed to understand the functional properties of actin filament assembly in the nucleus for chromatin organization and cell division. Actin filament assembly in the cytoplasm has well-characterized roles for cellular movement and contraction, but not much is known about its functions inside the nucleus. During the first period of our Program Grant project, we discovered that actin filaments are transiently assembled inside daughter cell nuclei after cell division at mitotic exit, which is required for chromatin decompaction and nuclear volume expansion (1, 2).
These findings prompted us to test the role of actin assembly during pronuclear formation when early embryonic nuclei grow in size. Interestingly, nuclear actin is assembled to form filamentous, even network-like structures in pronuclei of fertilized mouse embryos during the one-cell stage (see figure). Our studies indicate that nuclear actin polymerization controls DNA damage repair on parental DNA, which is necessary for fertilized embryos to develop normally. Our findings unveil an important developmental role for actin assembly inside the nucleus.


































