Birds of a feather may flock together, but the feathers of birds differ altogether in different birds and at different developmental stages. For example, downy feathers and semiplumes primarily play a role in thermal regulation, remiges and rectrices are modified for flight, whereas contour feathers streamline the body suface and are patterned for display and/or camouflage while helping to protect against the loss of body temperature. Despite such diversity, extant feathers share a common core design: a typical vaned feather is characterized by a main shaft, called the rachis, fused by a series of barbs on each side. The barbs themselves are further fused on each side by a third series of branches, called barbules. This three-level hierarchical-branching structure allows feathers to meet the requirements of multiple functions at the same time, such as waterproofing, thermal insulation, sexual display, streamlining the body surface, generating lift, and transmitting thrust, while their cyclical regeneration enables rapid shifts in response to environmental changes.
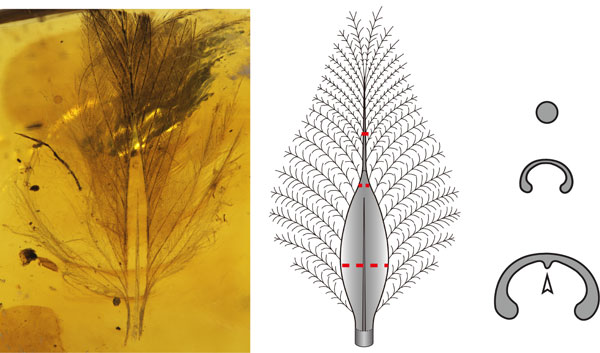
Figure: A feather embedded in Burmese amber with a ventrally-open shaft (left) and schematic drawings showing the anterior and cross-section views of the feather shaft (right).
As the basic level of the hierarchical-branching structure, the shafts of most extant feathers are cylindrical, composed of a dense cortex and spongy internal medulla, which maintain a certain range of strength while being light weight. In our HFSP collaboration, we discribed three bizarre morphotypes of primitive feather shafts present in the ~99 million-year-old Burmese ambers, which are not cylindrical as typical extant feather shafts but are ventrally-open and crescent-shaped in a cross-section view. This raises the question of how the cylindrical extant feather shaft evolved and whether the ventrally-open crescent-shaped shafts are unique to primitive feathers.
By investigating the developmental processes involved in forming the cylindrical extant feather shafts, we found that their tissue proliferation and differentiation follow a similar order, from the dorsal side to the ventral in cross-section view. When comparing the amber embedded feathers to a variety of stages of the developing cylindrical feather shaft, we were suprised to find that the ventrally-open crescent-shaped feather shafts are developmentally equivalent to a variety of immature cylindrical feather shaft stages. This suggests the bizarre ventrally-open feather shafts might be non-well-differentiated cylindrical shafts, which supports the idea that extant feather complexity evolved through the incremental, stepwise acquisition of derived characteristics. Functional simulations suggest that feathers with ventrally-open shafts are much weaker than cylindrical ones, and the increased mechanical strength is likely to have been a key factor that drove cylindrical feather shaft evolution.
This finding systematically reveals the evolutionary history of the cylindrical extant feather shaft. It is a complementary study to the paper published last year (Chang, Wu et al. 2019), in which we demonstrated that the primitive contour feathers form vanes by overlapping adjacent barbs but not by connecting proximal and distal barbules with hooklets, which indicates that other levels of the hierarchical-branching structure of extant feathers have also evolved stepwise.


































