HFSP grant awardee Valentina Emiliani and her colleagues achieved millisecond action potential induction with sub-millisecond precision for neurons in the living mouse visual cortex by applying the novel light-shaping technology pioneered in her laboratory. An illumination volume matching the size of one or multiple neuronal soma up to 300 µm deep in the mouse cortex is simultaneously created via computer-generated holography with temporally focused laser beams of amplified pulse energy. This approach enables fast and precise two-photon optogenetic neuronal activation of opsins of diverse channel kinetics 1. These results provide the first step to in vivo optical interrogation of neuronal wiring and its role in brain functions.
Millisecond control of excitability in one or multiple neurons in the living brain is invaluable for its study and remedy. Optogenetics brings about tremendous opportunities for optical control of spike generation by shining light onto neurons expressing light-sensitive ion channels, opsins 2,3. Widefield illumination of visible light in combination with genetic targeting of opsin expression in predefined cell subtypes has added a certain degree of specificity to brain cell activation 4,5. However, to probe the wiring and the heterogeneous roles of individual neurons within the same neuronal subpopulation, the ability to focus light onto one or several selected cells through the scattering brain tissue is critical. Two-photon (2P) excitation, providing excellent optical sectioning, is a promising solution to combat tissue scattering.
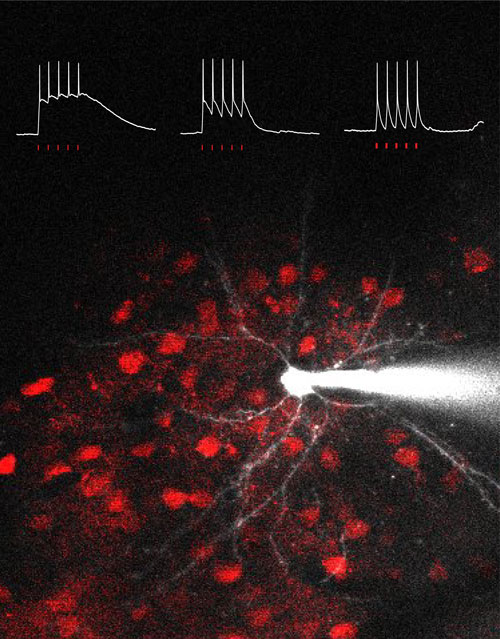
Figure: Three intracellular recordings indicate membrane potential responses upon a train of five holographic illuminations onto example target cells expressing opsins ReaChR, CoChR, or ChrimsonR. The background image shows ReaChR-positive cells in red and one is targeted by a patch microelectrode.
Innovative optical methods of scanning or parallel approaches, in combination with new-generation opsins and amplified laser sources, have allowed 2P optogenetic neuronal activation in a spatiotemporally precise manner in the scattering brain tissue 6. Briefly, the scanning approach swiftly deflects the laser beam in a trajectory covering the target soma for actuating opsins sequentially 7–9. The parallel approach, on the other hand, generates a light-pattern illuminating the target area at once 10–15. The advantage of the parallel method is simultaneous actuation of all opsins under the desired illumination-pattern, thus permitting fast and precise neuronal activation for opsins of a wider range of channel kinetics 1,16–18.
The interdisciplinary team of HFSP Program Grant holder Valentina Emiliani at the Vision Institute in Paris has pioneered 2P optogenetic activation by developing optical methods including computer-generated holography (CGH) and temporal focusing (TF) to shape light into the brain 19,20. Recently, Valentina Emiliani’s team further achieved un-biased single-cell activation by combining parallel light-shaping and soma-targeted opsin expression 17, opening up opportunities of optical probing of neuronal connectivity.
In brief, the principle of CGH consists of computing, using a Fourier transform based iterative algorithm, the interference pattern or phase-hologram that light propagating from a defined target (input image) would form with a reference beam on a defined “diffractive” plane. The computer-generated phase-hologram is then addressed to a liquid-crystal matrix spatial light modulator (SLM) placed at the diffractive plane. Illumination of the screen with the laser beam (or reference beam) will generate at the objective focal plane a light pattern precisely reproducing the shape of the target.
TF effectively improves the axial confinement of generated holographic patterns in the scattering tissue by compressing the laser pulse-width at the focal/sample plane and smearing out above and below, thus ensuring 2P excitation occurs at the focal plane. In principle, a diffraction grating is placed after the SLM and conjugated with the focal plane to first expand the laser beam in the color dimension and then recombine the different wavelength components at the sample plane.
Until today, only a few studies reported in vivo 2P optogenetic activation 8,9,13,14,21, without sufficient details of its temporal characteristics. In this work1, I Wen Chen, Emiliano Ronzitti and their colleagues applied the custom-developed light-shaping technology of TF-CGH for in vivo spike generation for three opsins of different channel closing time constants: ReaChR of ~100 ms 22, CoChR of ~30 ms, and ChrimsonR of ~15 ms 23. Using 2P-guided patch recordings in the layer 2/3 neurons of anesthetized mouse visual cortex, the team found the illumination conditions enabling action potential induction of <10 ms peak latency and <1 ms jitter for the 3 opsins. Regular or irregular spike trains can be precisely induced by repetitive illuminations onto the target neuron. They further demonstrated multi-cell activation in an all-optical manner by simultaneous holographic illumination onto multiple target cells while monitoring calcium signals in a neuronal population expressing both opsins and the calcium indicator GCaMP6 in anesthetized and awake mice. These results are summarized in a research article published in May 2019 in the Journal of Neuroscience 1.
Emiliani's team is now working towards probing the relationship between neuronal wiring and functional properties, e.g. visual selectivity, by incorporating soma-targeted opsins and 3D light-shaping in the in vivo setting. Taken together, the parallel holographic light-shaping provides unprecedent spatiotemporal resolution and precision for brain circuit manipulation 24,25.
The HFSP grant has been and will continue to be essential in attracting collaborations and bringing together the complementary skills of wavefront light-shaping, molecular engineering of opsins, and neurophysiology in Valentina Emiliani’s group.


































