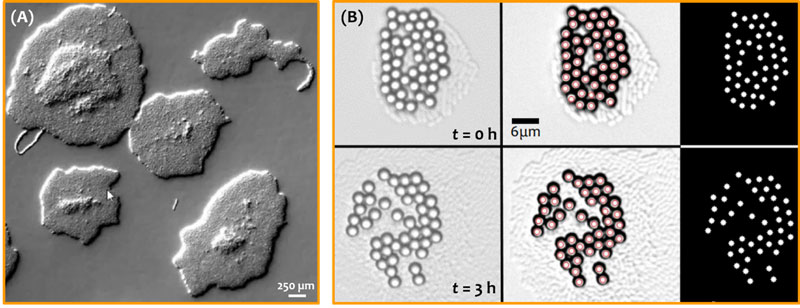
Figure. Structural transition – from single to multiple layers in nascent biofilms – is precisely timed due to self-regulation of phenotypic noise. Growing bacterial colonies organize as single, double or triple layers of cells, visualized as elevated regions in panel (A). Structural transitions (single to double; double to triple layers, and so on) are followed by synchronous transport around the colony. The emergence of active transport is captured by the displacement of micron-sized tracers over time (panel (B), imaged over a duration of 3 hours). The growing bacterial colony can be faintly seen below the tracer layer (Copyright: Anupam Sengupta, Physics of Living Matter Group, University of Luxembourg).
Bacterial biofilms, found on nearly every natural and artificial surface that we come across, are crucial for human existence. Exquisite physico-chemical feedback mechanisms underpinning bacterial behaviour and physiology enable their prolific surface-associated lifestyles spanning the human gut to plant and soil environments. Yet how variability in cell-level biophysical traits – so far unaccounted for in living and active matter models – impacts emergent collective phenomena has remained a challenge, and thus largely unexplored. Now Prof. Anupam Sengupta (former HFSP Cross-Disciplinary fellow) and his team at the Physics of Living Matter Group, University of Luxembourg, have discovered how biofilms – and living systems in general – regulate the timing of collective events emerging from individuals with highly variable and dynamic attributes. Lead author, Dr. Jayabrata Dhar, also an HFSP Cross-Disciplinary fellow in Prof. Sengupta’s Lab, co-developed a quantitative pipeline to extract the phenotypic noise across different traits, and estimated the emergent hydrodynamic flows around expanding bacterial colonies. Taking a quantitative biophysical approach, the cross-disciplinary team zoomed into nascent bacterial biofilms, demonstrating that the timing of the structural and hydrodynamic transitions – key developmental steps of growing biofilms – emerges collectively due to the self-regulation of individual traits. The self-regulated phenotypic noise across various parameters (e.g., cell aspect ratio and cell division time) cross-talks to mitigate each other’s random effects, ultimately enabling a well-timed execution of the structural transition of 2D layers into 3D-morphology. Such structural transitions drive synchronous active flows, which are strong enough to transport biologically relevant micro-cargo.
The study, conducted on different strains of Escherichia coli and Serratia marcescens growing under diverse conditions (nutrient levels and temperatures), forges the first direct, mechanistic link between activity-dependent variability at individual-scale and population-scale emergent properties in living systems. Remarkably, despite the species being non-motile, they could generate active hydrodynamic flows which could be finely regulated by tuning the growth temperature or the nutrient levels. The local flows colony micro-environment was strong enough to disrupt self-assembled particle clusters (used as tracers, see figure panel B), and shuttle them actively across the microbial environment. The authors adopted a cross-scale and cross-disciplinary approach to understand the role of phenotypic noise, employing a combination of single-cell time-lapse imaging, particle image velocimetry, numerical simulations and continuum modelling tools. The synchronous enhancement in transport – in an otherwise diffusion-limited setting – by more than two orders of magnitude suggests biological functions of such active flows in the transport of molecular and micro-cargo during the early stages of biofilm development. Expanding colonies exhibit strain- and activity-dependent phenotypic noise, yet, crucially, this work reveals that the 2D to 3D morphological transitions are insulated from noise, rendering it a statistically precise process in the life of a sessile colony.
Looking forwards, Prof. Sengupta’s team aims to understand how sessile colonies harness these well-timed collective events to tune inter-colony communication under stressful environments, for instance those associated with the dysbiotic gut environments or microbiome associated with specific cancer types. To read more about the work, please see the press release from the University of Luxembourg.
Link to the Physics of Living Matter Group at University of Luxembourg
|
HFSP award information Cross-Disciplinary Fellowship (LT000368/2019-C): The microscale biophysics of toxin dispersion during harmful algal blooms Fellow: Jayabrata Dhar |
|
HFSP award information Cross-Disciplinary Fellowship (LT000993/2014-C): The biophysics of microbial nutrient uptake in flow Fellow: Anupam Sengupta |


































