Eukaryotic cilia and flagella are appendage-like organelles that play critical roles in cell motility, sensing and development. The axoneme, which serves as a scaffold structure of the cilia, is a microtubule-based structure that has a unique architecture of “9+2”: nine peripheral doublet microtubules that surround a central pair of singlet microtubules (see figure below).
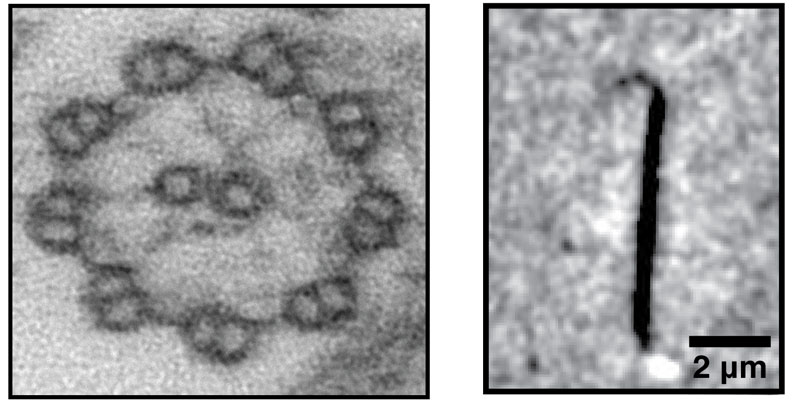
Figure: Axonemal microtubules - Left: Axoneme structure with 9+2 architecture: nine doublet microtubules in the periphery and the central pair composed of two microtubules singlets in the center. Right: Reconstituted axonemal microtubule showing curved tip structure.
Strikingly, axonemal microtubules are in a steady state (no net growth or shrinkage). This is in contrast to cytoplasmic microtubules that are known as dynamic polymers whose ends switch stochastically between phases of slow growth and rapid shrinkage, in a process called dynamic instability. Moreover, axonemal microtubules show remarkable high mechanical strength, and unusually, stability against depolymerization, thus suggesting that there are differences between axonemal microtubules and cytoplasmic microtubules. However, the study of axonemal microtubules in vitro was impossible until recently since existing procedures used to solubilize microtubules during their purification do not work. Thus, leaving many open questions regarding the unique biochemical and biophysical properties of the axoneme.
In this study, we pave the way to answer many of these questions. We used the Hofmeister series to develop a meticulous method to solubilize tubulin building blocks from axonemal microtubules and to purify, for the first time, functional tubulin. Furthermore, we demonstrate the solubilization of tubulins from the central pair and from the doublet microtubules together and separately, while characterizing the post-translational modifications within the axoneme structure. We characterize the biophysical properties of the reconstituted axonemal microtubules in vitro and show that axonemal tubulin supports the high length stability of the axoneme by two distinct mechanisms: low dynamicity and high stability of the protofilaments, which form the microtubules.
Another part of this study is related to the unique growth behavior of axonemal microtubules. Our knowledge of the microtubule tip structure is based mainly on static electron microscopy studies, due to technical limitations to image the tip structure of dynamic microtubules using a light microscope. Therefore, there is uncertainty about the tip structure of dynamic microtubules. Interestingly, the high stability of the protofilaments of axonemal microtubules enabled us to observe, for the first time, remarkably long tip structures of dynamic microtubules (see figure above). We find that growing microtubules display outwardly curved ends with a low number of protofilaments.
Finally, a surprising finding of this study is related to tubulin diversity (also known as the tubulin code), which is composed of post-translational modifications (PTMs) and tubulin isotypes. Tubulin is subjected to various PTMs but their role in the dynamic properties of microtubules is still unknown since the multiple tubulin isotypes may provide another source of functional diversity. Therefore, in our study we used the biflagellate green alga, Chlamydomonas reinhardtii, that has single tubulin isotype. Consequently, we were able to conclude that PTM of polyglutamylation has no significant effect on the dynamic properties of the microtubules, despite the electrostatic charge.
While many other questions regarding the axonemal microtubules remain open, this is the first step in elucidating the underlying mechanisms. Their study in vitro will allow us in the future to gain new insights into the ciliary scaffold, PTMs and the growth of microtubules.


































