Two California-based HFSP Long-Term Fellows, Leonardo Morsut and Satoshi Toda, describe their highly innovative research and how they are using synthetic cell biology tools to engineer customized self-organizing tissues.
Using artificial cell-cell signalling circuits, we generated robust multi-layered self-organizing structures displaying cell type divergence, symmetry breaking, and regeneration upon injury. Our work provides insights into the evolution of multicellularity and demonstrates a novel way to engineer customized self-organizing tissues or materials.
 |
Leonardo Morsut is Assistant Professor of Stem Cell Biology and Regenerative Medicine at the Keck School of Medicine at the University of Southern California (USC). He is also director of a new Center for Integrated Electronics and Biological Organisms (CIEBOrg) in the Biomedical Engineering Department at USC Viterbi School of Engineering. In 2012, he was awarded an HFSP Long-Term Fellowship to train in the lab of Wendell Lim at the University of California San Francisco where he developed a new class of synthetic receptors called Synthetic Notch receptors that have far-reaching implications for cell therapies for cancer, autoimmunity, and regenerative medicine. |
 |
Satoshi Toda is a postdoctoral fellow in Wendell Lim’s lab at the University of California San Francisco. He is interested in how the systems of molecular and cellular networks can achieve biological functions and how we can engineer the systems to output desired functions. He was awarded an HFSP Long-Term Fellowship in 2016. |
The development of complex tissues starting from less organized groups of cells requires that cells communicate with each other and make coordinated, collective decisions about when and how to organize themselves. Central to this tissue organization are cell-cell communication networks that induce changes of cell mechanical properties, like cell shape, cell-cell adhesion, etc. In this study, we used synthetic cell biology tools to create simple signalling circuits that modulate cell-cell adhesion. By manipulating and layering these circuits in otherwise naïve cells, we were able to generate two- and three-layer multicellular structures that display many of the hallmarks of developmental systems.
As the basis for our engineered circuits, we used Synthetic Notch, or SynNotch, transmembrane receptors. SynNotch receptors are a synthetic version of the Notch receptor, which enables the programming of both the input to the receptor and resulting output of the cell3. We engineered mouse fibroblast cells with a series of SynNotch based cell-cell communication circuits inducing the expression of different adhesion molecules of the cadherin superfamily as well as fluorescent reporter proteins (Fig. 1A). In the simplest circuit, we programmed two groups of cells to self-organize into a two-layered sphere. One group of cells acted as signal sender cells and expressed a ligand (CD19 protein) on their surfaces. These cells also expressed a blue fluorescent protein (BFP) for tracking purposes. The second group of cells acted as signal receiver cells and expressed a SynNotch receptor capable of binding to the sender cells’ CD19 ligand. In response to binding, these receiver cells activated both E-cadherin and a green fluorescent protein (GFP). In isolation, these two different groups of cells did nothing, but when the two groups were mixed, the sender cells activated SynNotch receptors on the receiver cells triggering them to produce E-cadherin and GFP. As a result of E-cadherin expression, receiver cells had higher affinity for each other than for sender cells and quickly clustered together. Within 24 hours, a symmetric two-layer structure emerged with a green central core made up of receiver cells surrounded by a blue exterior layer of sender cells (Fig. 1B).
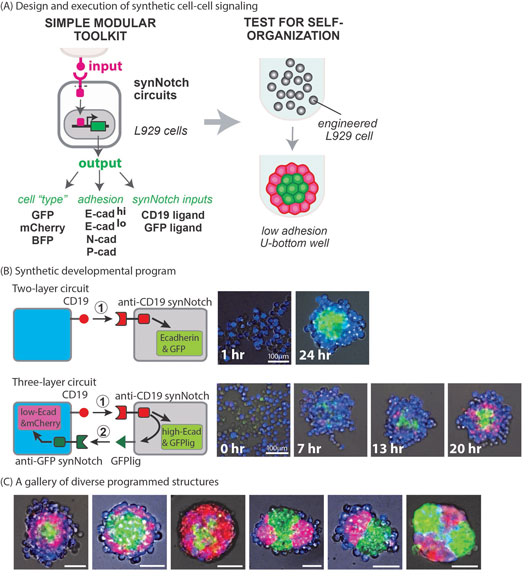
Figure 1: Engineered synthetic self-organizing program. (A) We have induced three types of outputs with SynNotch signalling to achieve synthetic self-organization; cadherin adhesion for cell sorting, fluorescent proteins indicating cell type and new SynNotch ligands for signalling cascades. (B) Synthetic cell-cell communication between sender and receiver cells that robustly generates two or three-layer spheroids. Two layer: receiver cells were activated by sender cells to express E-cadherin and GFP, resulting in a green core aggregate. Three layer: first cell-cell signalling induced E-cadherin in receiver cells to form a green core (13hr). Then green cells induced second signalling to neighboring blue sender cells to activate different cadherin and red signal, resulting in the sequential formation of a red middle layer (20hr). (C) By inducing combinations of different cadherins in the SynNotch circuit, diverse three-domain structures were self-organized.
By layering different synthetic cell-cell signalling circuits, we were able to increase the complexity of the resultant structures. In subsequent iterations, we modified receiver cells such that, in addition to expressing high levels of E-cadherin upon activation, they produced a cell-surface presenting GFP designed to act as a ligand for a second level of communication. We further modified sender cells to express, in addition to BFP and the CD19 ligand, their own SynNotch receptor capable of binding and interpreting the receiver cells’ new GFP ligand. In response to GFP ligand binding, the sender cells now expressed low levels of E-cadherin and a red fluorescent protein (mCherry). Again, in isolation, the two groups of cells did nothing. When mixed, blue sender cells activated receiver cells and triggered them to both cluster and express a GFP ligand. In turn, any blue sender cells in contact with any of these green cells were triggered to express mCherry and a low level of E-cadherin. This subset of previously blue cells expressed enough E-cadherin to be drawn towards the core, but not enough to remain within it. The result was a three-layer structure with a green core, an external layer of blue cells, and a thin layer of red cells sandwiched between them. In other iterations, we varied the cadherin type, which further influenced sorting behaviour. By altering the input-output characteristics of these simple circuits and introducing different adhesion molecules, we generated a wide array of both symmetric and asymmetric structures (Fig. 1C).
In order to test the robustness of the generated structures, we devised a way of both subjecting them to injury and recording their regeneration using time lapse videography. In collaboration with Lucas Blauch in Prof. Sindy Tang's Lab at Stanford University through a meeting in the NSF Center for Cellular Construction, we used microfluidics and a micro-guillotine to cut the structures in half and record the outcome over several hours. We found that the structures self-repaired and reformed, according to their programming.
While it was known that certain fibroblast cells undergo differential sorting when they express different cadherin molecules, our work was the first to use synthetic gene networks to drive sorting events. In embryonic development, genetic networks selected through evolution guide a small number of cells to self-organize to ultimately create the multitude of cell types and tissues in the adult body. In this work, we show that simple engineered cell-cell communication circuits can create complex multicellular structures capable of cell type divergence, symmetry breaking, and regeneration upon injury. Since these are the basic routines that all multicellular organisms display, it is tempting to speculate that the first multicellular forms had similar kinds of genetic circuits that enabled them to organize their component cells. Continued progress in this area could pave the way for a field devoted to 'synthetic development', where more complex synthetic networks can execute a variety of effector functions in cells, including more complex morphogenesis.
This work relied on the previous technological advancement of the programmable signalling molecule SynNotch, which was also published during Leonardo Morsut’s HFSP Fellowship in the Lim Lab. This technology allows scientists to reprogram cell communication and to customize both signals and responses. The SynNotch receptors were highlighted as notable advances in the 2016 year-end issue of Nature Medicine1, and the technology has attracted substantial interest from the academic world. SynNotch technology has also been patented and licensed to a start-up company (CDL, recently bought by Gilead Sciences) that is optimizing its function for medical applications in cancer immunotherapy (http://www.businesswire.com/news/home/20171207006250/en/).
HFSP funding was instrumental in this work for several reasons, both financial and motivational. As the former is more obvious, we would like to say something about the latter. For international postdocs, this phase of our careers is particularly stressful. We are trying to perform exciting research while figuring out what we want to do when we 'grow up' and move beyond the mentored phases of our careers. The HFSP Fellowship afforded a degree of psychological ease and recognition, which allowed us to focus on the kind of science that we wanted to do. It wasn’t stress-free, but the fellowship allowed us to embark on high-risk projects with less fear. The HFSP support provided the foundation to be competitive in the academic job market, and one of us, Leonardo Morsut, is now Assistant Professor in the Department of Stem Cell Biology and Regenerative Medicine at the University of Southern California in Los Angeles.
|
References [1] Gene therapy Keeping it precise. Stower, H. et al. Notable advances 2016. Nat. Med. 22, 1374–1376 (2016) [2] Self-organized multicellular structures programmed form engineered cell-cell signaling cascades. Toda S. et al.. Science 2018 May 31; eaat0271 (*co-last authors). [3] Engineering customized cell sensing and response behaviors using synthetic Notch receptors. Morsut L*, Roybal KT* et al. (2016). Cell 2016 Feb 11;164(4):780-91. Epub 2016 Jan 28. (*co-first authors) |


































