 |
Ryosuke Kojima is an assistant professor at the Graduate School of Medicine at the University of Tokyo and has a concurrent appointment as a JST PRESTO researcher. He obtained his PhD at the University of Tokyo in the lab of Prof. Yasuteru Urano in 2014. His postdoctoral research in Prof. Fussenegger's laboratory at ETH Zürich was supported by an HFSP Long-Term Fellowship. In 2019, he was awarded an HFSP Career Development Award to support his research at the University of Tokyo following his return in 2017. His research interests are in the area of synthetic biology and chemical biology. |
Engineered immune cells that can sense specific cell contacts are useful for cancer therapy (e.g., chimeric antigen receptor T-cells). However, the use of engineered immune cells involves risks of cytokine release syndromes. Further, engineered-immune-cell-based cancer therapies have so far only been successful for a limited range of cancers, due to the difficulty in controlling their migration properties.
One of the solutions available for overcoming these problems would be to engineer non-immune cells that are inherently tumor tropic for cancer cell ablation. Indeed, for example, mesenchymal stem cells (MSCs), that are reported to be inherently tumor tropic, have been engineered to constitutively express an enzyme to convert an anti-cancer prodrug into a toxic metabolite for cell-based enzyme-prodrug cancer therapy. Taking possible toxicity into consideration, it would be better to convert the prodrug into the active metabolite only around cancer cells. However, it has so far been difficult to make non-immune cells capable of sensing specific cell contacts, due to the shortage of available design principles to make mammalian cells responsive to specific cell contacts (T-cell signaling is highly specialized, so adapting it to non-immune cells is difficult). Herein, we have developed a new class of signaling device that endows non-immune cells with the ability to sense specific cell contact, and secrete a therapeutic molecule accordingly.
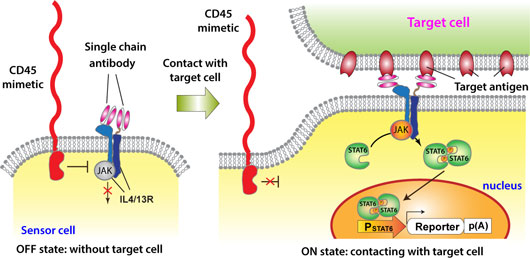
Figure: Synthetic-biology-inspired signaling device for sensing specific cell contact. A sensor cell expresses interleukin 4 / interleukin 13 receptors (IL4/13R) bearing extracellular antigen recognition moieties. Without a target cell, activity of JAK associated with IL4/13R is inhibited by a CD45 mimetic in close proximity. When the sensor cell recognizes a target cell, the CD45 mimetic is segregated from the cell-cell interface due to its large extracellular domain. As a result, JAK is liberated from the suppression by the CD45 mimetic, which triggers the downstream signaling.
To design a cell-contact-sensing signaling device that is functional in non-immune cells, we focused on the following two criteria: (1) when a T cell recognizes a target cell, a transmembrane phosphatase called CD45 expressed on the T cell is physically segregated from interface between the T cell and the target cell. Then, CD45 becomes unable to suppress a signal initiating kinase at the cell-cell interface due to inaccessibility, which triggers downstream T-cell signaling[1]; (2) CD45 also suppresses JAK-STAT pathways that are functional in a variety of non-immune cells[2]. We hypothesized that OFF/ON switching of a JAK-STAT signaling pathway might be controllable by segregation of CD45 upon cell-cell contact, which would enable non-immune cells to sense specific cell contacts. In our paper recently published in Nature Chemical Biology, we successfully showed that this signaling-device design indeed works.
Furthermore, we applied designer non-immune cells equipped with the cell-contact-sensing device to cell-based cancer therapy. More concretely, we endowed non-immune cells capable of expressing a prodrug converting enzyme (as mentioned above) with a target sensing ability by using the cell-contact-sensing signaling device, and applied them to target-specific cell ablation (please check the original paper for more details!).
Thus, in this study, we have developed a new class of cell-contact-sensing devices that has the potential to become useful in future cancer therapy. This study also contributes to the advancement of synthetic biology by providing a new design principle to transmit extracellular information into cells. At present, it is not easy to stably integrate multiple gene components into the sensor cells in a controlled manner. However, as gene editing technologies improve, precise quality control of the designer cells should become possible. Also, if we can unveil how this system functions in living animals (ultimately in the human body), it might become possible to apply the designer cells (or design principles of cellular functions) to medical applications in the future.
|
Acknowledgement to HFSP My goal is to meet unmet medical needs by revolutionizing advanced medicine. To achieve this, I believe new technologies to control complex biological systems are needed. I studied small-molecule-based chemical biology during my PhD study. This gave me insight into the versatility of small molecules as biomodulators. However, at the same time, I came to realize that a small-molecular approach is sometimes insufficient to control complex biological systems, and I thought an expansion of my interests into synthetic biology would offer new possibilities for achieving precise control of complex systems. Therefore, I joined the laboratory of Professor Martin Fussenegger, an expert in the field of mammalian synthetic biology, as a postdoc. I decided to work on engineering mammalian cell-to-cell communication, since I believe multicellular communication is indispensable for controlling complex biological systems. That is how the published project started. Without the support of the HFSP Fellowship, which encourages us to bravely change research fields, I would have not been able to jump into this totally new field. I am grateful for the huge support of HFSP. |


































