In 1896, two German scientists, Köttgen and Abelsdorff, made a curious observation: when they measured the absorption properties of visual pigments (the molecules that mediate light sensation) extracted from the eyes of freshwater fish, they found them to be ‘red-shifted’ towards longer wavelengths than those of marine fish and land animals. The significance of this seemingly obscure discovery was not appreciated until nearly 40 years later, when George Wald took up the problem. Wald, who later won a Nobel Prize for his work on the visual system, found that the red-shift of the freshwater fish’s visual pigment was caused by a simple chemical modification in the pigment. Wald noted that this chemical modification was absent from the visual pigments of marine fish and land animals. The visual pigments of vertebrates consist of two parts: a protein component called the ‘opsin’, and a small molecule, retinaldehyde, which is bound to the opsin. Wald found that the retinaldehyde in the eyes of freshwater fish contained an additional conjugated double bond that caused the molecule to preferentially absorb light of longer wavelengths. To distinguish between the form of retinaldehyde found in freshwater fish and that found in other animals, Wald referred to the former as ‘vitamin A2’ and the latter as ‘vitamin A1’. Wald believed correctly that the red-shift caused by replacing vitamin A1 with vitamin A2 would permit freshwater fish to peer more deeply into their murky, red-shifted aquatic environment.
 |
Dr. Joseph C. Corbo, MD, PhD, is an associate professor in the Department of Pathology and Immunology at Washington University School of Medicine, in St. Louis, USA. He is a practicing neuropathologist, and his laboratory studies the development, diseases, and evolution of photoreceptors in the retina. He is a previous HFSP awardee of a grant entitled, “Deconstructing avian cone photoreceptors: a system of self-organizing optical microdevices”. |
 |
Dr. Nicholas Roberts, PhD, gained his first degree in Physics and Astrophysics at the University of Manchester, UK, where he also completed a PhD focused on optical studies of model biological liquid crystal systems relating to vertebrate photoreceptors in the Liquid Crystal Physics Group with Prof. Helen Gleeson in 2003. Much of the biological research was carried out at the University of Victoria, Canada. He continued splitting his time between the new Photon Science Institute at Manchester University, Queens University in Canada and the Ecology of Vision Group in Bristol. He is currently Director of Research of the School of Biological Sciences at the University of Bristol. |
Subsequent studies throughout the 20th century showed that a wide range of freshwater fish, amphibians, and reptiles use vitamin A2 to red-shift their visual system. The reason is simple: freshwater environments such as lakes and streams are often very murky, and the murkiness causes a red-shift in the light available for vision. The animals therefore dynamically adapt their visual system to match the wavelengths of light in their environment. A classic example of this adaption occurs during the migration of salmon: when salmon are in the open ocean (where the light is blue-green), they use vitamin A1. However, when the fish migrate into inland waterways to spawn, they switch to vitamin A2 to match their new red-shifted environment. The existence of this switch has been known since Wald’s early work in the 1930s, but the identity of the enzyme that converts vitamin A1 into A2 remained a mystery.
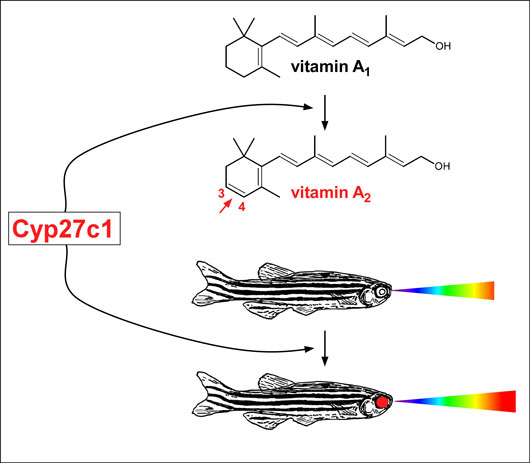
Figure 1
In November 2015, a team of scientists led by HFSP grantee Joseph Corbo, and including his HFSP co-investigator Nicholas Roberts, reported the discovery of the enzyme that mediates the vitamin A1 to A2 switch. This enzyme, called Cyp27c1, is a member of a large family of enzymes known as ‘cytochrome P450s’. The scientists found that by treating zebrafish with thyroid hormone, they could induce the switch from vitamin A1 to A2. This finding permitted the scientists to compare the gene expression profiles of thyroid-treated zebrafish with controls to identify the gene encoding Cyp27c1 (Figure 1). In parallel, the scientists also used a second model system to find the enzyme, the American bullfrog. Bullfrogs like to sit at the surface of ponds, scanning the red-shifted underwater environment while simultaneously monitoring the aerial environment. George Wald and colleagues showed in 1971 that the upper eye of bullfrogs contains vitamin A2 (presumably to facilitate downward vision into the murky water), whereas the lower eye contains only vitamin A1 (Figure 2). Corbo and colleagues profiled gene expression in the upper and lower eyes of bullfrogs, finding that the gene encoding Cyp27c1 was expressed in the upper part only. Thus, both fish and amphibians appear to use the same enzyme to enhance their sensitivity to long-wavelength light. Additional studies of Cyp27c1 in zebrafish showed that the enzyme is both necessary and sufficient for the production of vitamin A2 and required for red-shifting the sensitivity of the visual system. Thus, this enzyme is critical for the ability of these species to dynamically enhance their visual sensitivity to long-wavelength light, including near-infrared light.
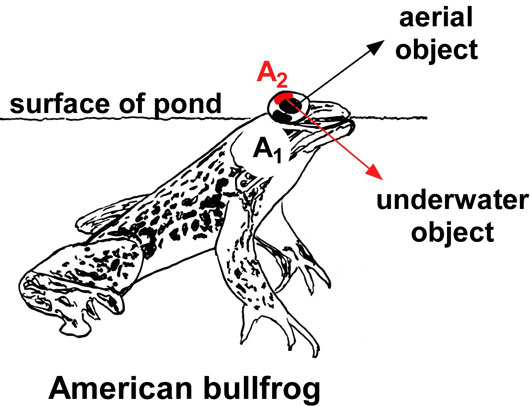
Figure 2
Although this research was conducted under the auspices of an HFSP grant, it was not part of the original grant proposal! The goal of the original grant was to decipher the mechanism underlying the unique optical adaptations of the avian cone photoreceptors of birds. One aspect of the bird studies involved the analysis of the pigment content of cone oil droplets, which are small optical organelles that act as spectral filters on incoming light. These oil droplets are pigmented with carotenoids, the chemicals that endow many plants and animals with bright yellow, orange and red colors. The spectral properties of the oil droplet carotenoids are tuned by the selective addition or subtraction of conjugated double bonds, in a manner very similar to the way in which vitamin A1 is chemically modified to form vitamin A2. Thus, the studies in birds led the research team to be interested in the old problem of identifying the enzyme that mediates the vitamin A1 to A2 switch in fish and amphibians. This tangential interest, sparked by work in birds and funded by HFSP, ultimately led to the discovery of Cyp27c1 and the solution of a longstanding mystery in vision science.
This scientific story underscores the critical importance of the kind of curiosity-driven, blue-sky research supported by HFSP, and it also highlights the highly nonlinear, serendipitous nature of scientific advances. Would a disease-oriented funding agency such as the NIH fund a study aimed at characterizing an enzyme localized exclusively to the upper eye of bullfrogs that enhances the animal’s ability to gaze down into murky water?! Probably not. And yet, it turns out that Cyp27c1 has major implications for biomedicine. The research team is now pursuing several applications of Cyp27c1 in optogenetics, a body of techniques for controlling cellular activity with light. Since longer wavelengths of light penetrate tissue more deeply, the field of optogenetics has worked hard to create optogenetic devices with red-shifted spectral properties. The team is coexpressing Cyp27c1 with optogenetic devices to red-shift their function into the infrared range. This work includes efforts to create new optogenetics therapies for blindness as well as an ‘all-optical’ cardiac pacemaker. None of this would have been possible without the generous, flexible, and open-ended support of HFSP.
|
Reference Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting vitamin A1 into A2. Enright, J.M., Toomey, M.B., Sato, S.-Y., Temple, S.E., Allen, J.R., Fujiwara, R., Kramlinger, V.M., Nagy, L.D., Johnson, K.M., Xiao, Y., How, M.J., Johnson, S.L., Roberts, N.W., Kefalov, V.J., Guengerich, F.P., and Corbo, J.C. |


































