Since the discovery of Channelrhodopsin [1] and the first demonstration of photo-evoked action potentials in mammalian cells [2], optogenetics is progressively revolutionizing neuroscience research, opening up incredible opportunities in the field of medical research over the long term, from the treatment of neurodegenerative diseases and depression to the reestablishment of vision.
So far, most optogenetic experiments have used relatively simple illumination approaches that involve illuminating a large region of the brain with visible light. In spite of the lack of specificity of this illumination, this approach made it possible to control the activity of a specific network of neurons thanks to the precision of the genetic targeting. However, neurons that belong to the same neural network may have different neural codes and functions, such as being responsible for different tasks, responding to different stimuli, or having a particular activity pattern, thus requiring the development of new stimulation approaches capable of millisecond temporal precision and single cell resolution.
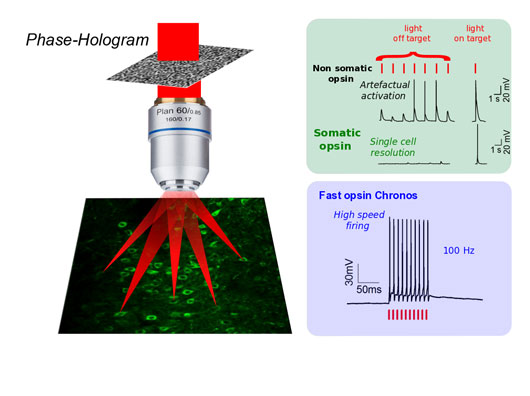
Figure: Holographic light modulation can reshape light to precisely target a single or multiple neuron(s). Using somatic opsins, such as soCoChR, enables a true cellular resolution to be reached by restraining opsin expression to the cell soma. Using fast opsins, such as Chronos, enables neuronal activity to be controlled with millisecond resolution and sub-millisecond precision even at high spiking rates.
Valentina Emiliani's team at the Neurophotonics Laboratory in Paris, France and Ed Boyden's laboratory at MIT in Boston, USA, have recently developed a new approach that allows spatial and temporal control of neuronal activation at a single cell level with a millisecond temporal resolution by combining two innovative techniques: a new targeting strategy for opsins that allows them to be confined to the neuron's cell body, and a two-photon microscopy technique based on the principle of computer generated holography (CGH), that enables the "sculpting" of light to target a single or a specific set of neuronal cell [3].
Briefly, the principle of CGH consists of computing with a Fourier transform based iterative algorithm the interference pattern or phase-hologram that back propagating light from a defined target (input image) will form with a reference beam, on a defined “diffractive” plane. The computer-generated phase-hologram is then addressed to a liquid-crystal matrix spatial light modulator placed at the diffractive plane. Illumination of the screen with the laser beam (or reference beam) will generate at the objective focal plane a light pattern precisely reproducing the shape of the target.
In the past years, Valentina Emiliani's team has demonstrated that two-photon excitation combined with CGH is effective for focusing laser light in order to obtain precise illumination of a single cell or a group of cells in the brain [4,5].
Despite the precision obtained with the holography technique, it was difficult to obtain optogenetic activation with true cell resolution. The explanation lies in the fact that the cell bodies of the neurons are densely surrounded by extensions called neurites (axons and dendrites) emanating from neighboring cells. Despite the optical confinement of the excitation volume, the illumination of the cell body of a single neuron can also excite those neurites that pass through the illumination volume, thereby causing parasitic excitation of neighboring neurons.
In order to solve this problem, Ed Boyden's team showed that the fusion of the opsin CoChR [6] to a short amino-terminal segment of the kainate receptor subunit KA2 allowed the selective trafficking of CoChR towards the cell body of the neurons of the cortex, thus avoiding the expression of opsins in the neurites.
Thanks to the combination of holographic illumination and this new protein, called somatic CoChR (soCoChR), both teams were able to highlight the stimulation of individual neurons in brain slices with millisecond temporal precision. They have also demonstrated that it is possible, with this approach, to measure the responses of a specific neuron to the stimulation of neighboring cells and thus detect its connections. This paves the way for a map of connectivity in the brain and the ability to analyze changes between real-time neural connections, when performing tasks, or learning new skills. This study was published in November 2017 in Nature Neuroscience1.
In the same year the two groups also combined two-photon holographic illumination with the fast opsin Chronos, a highly blue-light sensitive opsin discovered by the group of Ed Boyden in 2014 from the algae Stigeoclonium helveticum [6]. They demonstrated that the efficient current integration under holographic shaped light illumination enables action potential generation with sub-millisecond temporal precision and neuronal spike frequencies up to 100 Hz. Importantly, they also demonstrated that by using a fiber-amplifier, high-energy pulse laser enables average illumination power to be reduced drastically, opening the way for simultaneous targeting of hundreds of cells. This study was published in October 2017 in the Journal of Neuroscience2.
Currently, researchers are planning the transition to this type of study through the application of the method to the living animal. They are also working to improve the targeting of the molecule, to generate a somatic variant for the opsin Chronos and to develop somatic opsins with high electrical currents enabling inhibition of neuronal activity with single cell precision.
This work, supported by HFSP, could never have happened without merging the complementary skills in molecular biology, neurophysiology, non-linear optics and wave front shaping of Boyden’s and Emiliani’s groups.
References
[1] Temporally precise single-cell-resolution optogenetics. O.A. Shemesh, D. Tanese, V. Zampini, C. Linghu, K. Piatkevich, E. Ronzitti, E. Papagiakoumou, E.S. Boyden, V. Emiliani. Temporally precise single-cell-resolution optogenetics. Nat. Neurosci. 20 (2017) 1796–1806. doi:10.1038/s41593-017-0018-8.
[2] Sub-millisecond optogenetic control of neuronal firing with two-photon holographic photoactivation of Chronos. E. Ronzitti, R. Conti, V. Zampini, D. Tanese, A.J. Foust, N. Klapoetke, E.S. Boyden, E. Papagiakoumou, V. Emiliani. J. Neurosci. 37 (2017) 10679 –10689. doi:10.1523/JNEUROSCI.1246-17.2017.
Other references
[1] Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. G. Nagel, T. Szellas, W. Huhn, S. Kateriya, N. Adeishvili, P. Berthold, D. Ollig, P. Hegemann, E. Bamberg. Proc. Natl. Acad. Sci. U. S. A. 100 (2003) 13940–5. doi:10.1073/pnas.1936192100.
[2] Millisecond-timescale, genetically targeted optical control of neural activity. E.S. Boyden, F. Zhang, E. Bamberg, G. Nagel, K. Deisseroth. Nat Neurosci. 8 (2005) 1263–1268. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop....
[3] Dynamic holographic optical tweezers. J.E. Curtis, B.A. Koss, D.G. Grier. Opt. Communi. 207 (2002) 169.
[4] Two-photon excitation in scattering media by spatiotemporally shaped beams and their application in optogenetic stimulation. A. Begue, E. Papagiakoumou, B. Leshem, R. Conti, L. Enke, D. Oron, V. Emiliani., Biomed Opt Express. 4 (2013) 2869–2879. doi:10.1364/BOE.4.002869 197441 [pii].
[5] Three-dimensional spatiotemporal focusing of holographic patterns. O. Hernandez, E. Papagiakoumou, D. Tanese, K. Fidelin, C. Wyart, V. Emiliani. Nat. Commun. 7 (2016) 11928. doi:10.1038/ncomms11928.
[6] Independent optical excitation of distinct neural populations. N.C. Klapoetke, Y. Murata, S.S. Kim, S.R. Pulver, A. Birdsey-Benson, Y.K. Cho, T.K. Morimoto, A.S. Chuong, E.J. Carpenter, Z. Tian, J. Wang, Y. Xie, Z. Yan, Y. Zhang, B.Y. Chow, B. Surek, M. Melkonian, V. Jayaraman, M. Constantine-Paton, G.K.-S. Wong, E.S. Boyden. Nat. Methods. 11 (2014) 338–346. doi:10.1038/nmeth.2836.


































