During embryo development, tissues are remodeled to give shape to life. Seven is the number of fundamental tissue shape changes responsible to reshape an embryo. A tissue can (i) grow, (ii) shrink, (iii) twist, (iv) thicken, (v) thin, (vi) fold and (vii) extend. In the last decades, scientists have strived to identify the fundamental cell mechanisms responsible for these epithelial shape transformations. This knowledge is key to identifying the cause of fetal illnesses (e.g., spina bifida) to eventually develop effective therapies. Specialized mechanisms that drive specific tissue shape change have been identified. For instance, epithelial cells reduce their apical surface (a process known as ‘apical constriction’) in a coordinated fashion to initiate tissue folding. During tissue extension, cells remodel their contact junction exchanging neighbors in a stereotyped fashion (process known as ‘cell intercalation’). Nevertheless, during embryo development, a tissue can undergo multiple changes in shape not only sequentially but also simultaneously. For instance, during gastrulation the embryo of a vertebrate elongates along the anterior-posterior axis to separate the future head from the anus while it folds forming the neural tube. This raises an important question: how can a tissue undergo multiple simultaneous shape transformations if each transformation is per se driven by a functionally specific cellular mechanism? In addition, which signaling pathways are controlling composite morphogenetic processes? In this study, Alphy John and Matteo Rauzi uncover a new mechanism shining new light on the signaling pathway responsible for simultaneous tissue folding and extension.
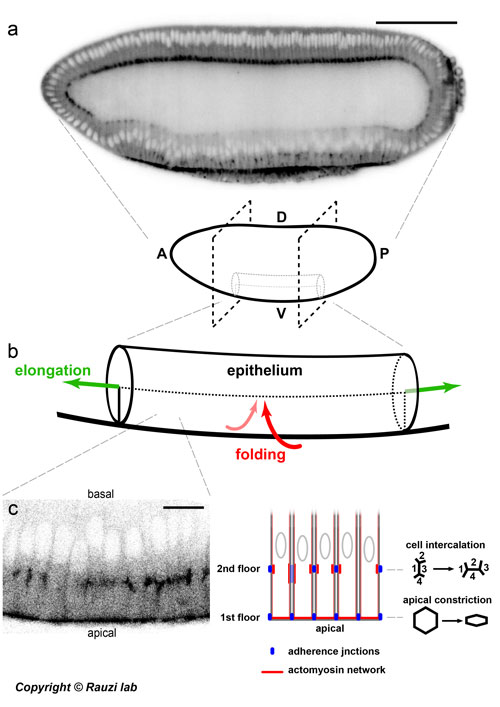
Figure: from the embryo to cell scale and back to unravel a two-tier junctional mechanism driving simultaneous tissue folding and extension.
(a) Drosophila embryo. Scale bar 100 µm. A - anterior, P - posterior, D - dorsal, V - ventral (b) The ventral tissue (the prospective mesoderm) folds along the ventral-dorsal axis and extends along the anterior-posterior axis. (c) The epithelial cells located on the ventral side establish a two-tier junctional mechanism driving simultaneous apical constriction and cell intercalation. Scale bar 10 µm.
The Rauzi team uses the Drosophila embryo (Figure a): an extremely powerful model system providing cutting edge genetic tools and free of restrictions to comply with ethical norms to study the fundamental cell mechanisms driving morphogenesis in a physiological relevant context. Adherens junctions are known to function as anchoring points in between cells on which forces, generated by coupled actomyosin networks, are exerted. In this study, Alphy and Matteo discover that epithelial cells can form distinct sets of adherens junctions at two different apical-basal positions resulting in a two-tier junctional system.
While the first junctional tier mediates apical constriction initiating tissue folding, the second tier mediates polarized cell intercalation initiating tissue extension (figure b and c). Alphy and Matteo show that the formation of two-tier junctions is under the control of the synergetic action of the anterior-posterior (AP) and dorsal-ventral (DV) gene patterning.
While AP and DV gene patterning has been studied and interpreted as sources of orthogonal signals eventually controlling separated features of a system, this study shows for the first time how these signaling sources can cross-talk and act synergistically to control morphogenesis during embryo development. Future work is now necessary to further explore the interplay between these fundamental signaling sources.
Matteo Rauzi, group leader at the University Côte d'Azur, mentioned that "the support of HFSP has been key to develop this scientific project that is, without any doubts, the best piece of science I have ever been involved in until today: without HFSP support this work would not have been possible."
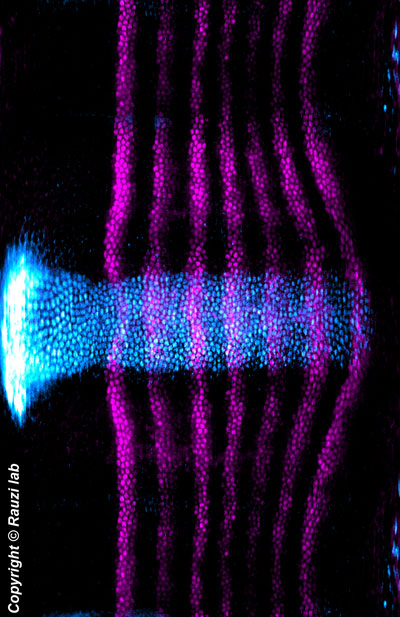
| Unrolled view of the embryo showing the expression pattern of the genes sna and eve. Ventral is in the center and dorsal is repeated at the at the top and at the bottom to emphasize the left-right symmetry of the embryo. |


































