Joint pain (arthralgia) and inflammation commonly occur with viral and bacterial infections (e.g., bacterial enteritis or streptococcal pharyngitis) and autoimmune diseases like lupus. This is often driven by circulating IgG immune complexes (IC), but why synovial joints are especially sensitive to systemic inflammation is unclear. The synovial membrane lines joints, housing blood vessels, nerves, and macrophages. Recent studies suggest immune cells and neurons communicate, but how circulating stimuli cause joint pain and the role of synovial immune cells remain unknown.
The HFSP Fellowship Awardee Tetsuo Hasegawa and team developed a method to dissect and image the entire mouse knee synovium, improving on past techniques using joint sections. Combining imaging, flow cytometry, RNA sequencing, and ex vivo cultures, they studied immune and neuronal responses to IC.
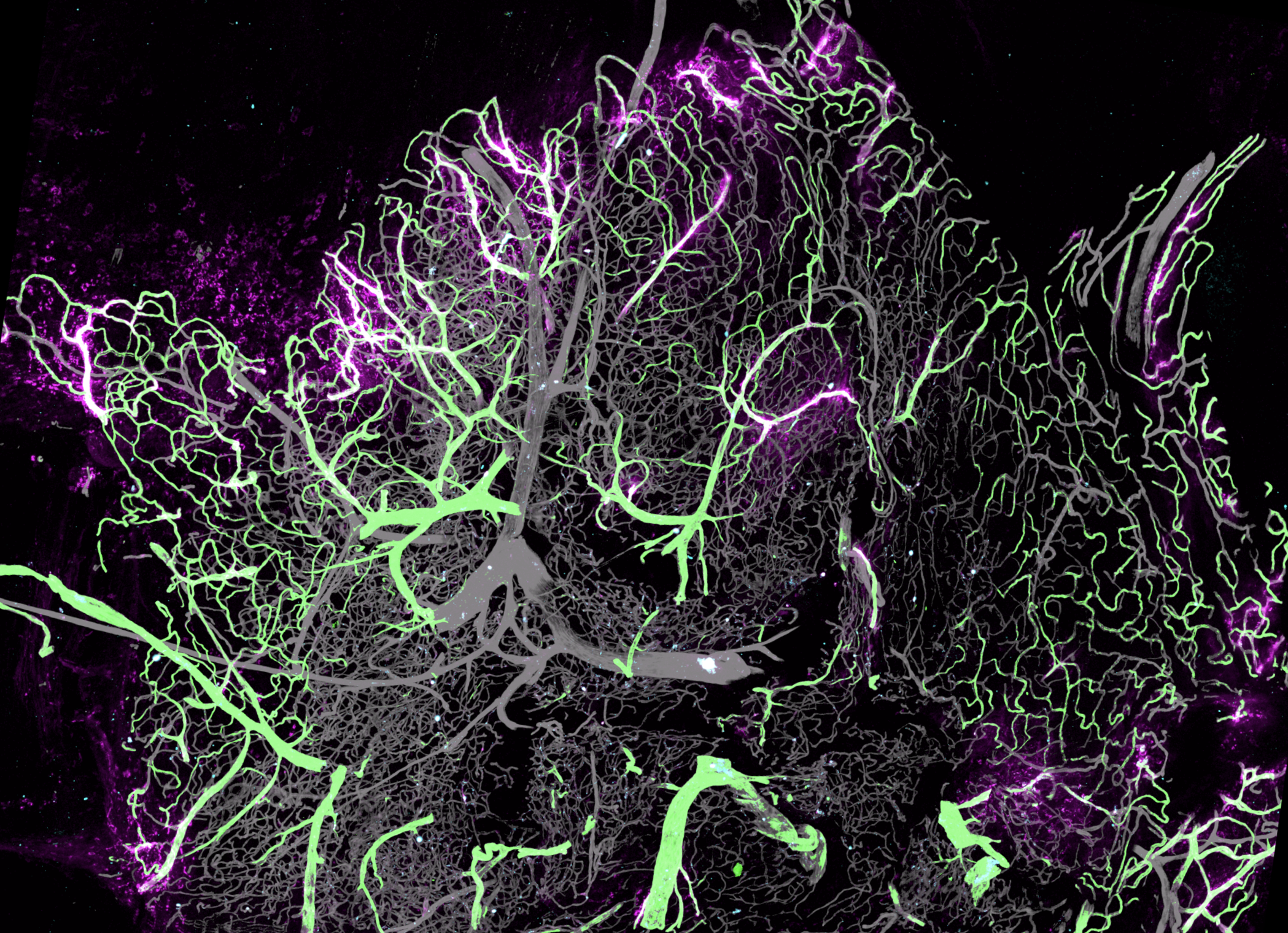
The researchers found that fenestrated PV1+ capillaries at the synovium's outer edge serve as entry points for circulating molecules. Lyve1+MHCII-negative macrophages cluster here alongside two MHCII+ macrophage subsets near these vessels. These macrophages respond differently to systemic IC: Lyve1+ cells release neutrophil-attracting chemokines and IL-1β, which activate CGRP-expressing neurons. In turn, neurons release CGRP, which enhances macrophage activity. This study reveals neuro-immune interactions at the blood-joint barrier that protect vulnerable synovial regions near fenestrated capillaries.


































