The protein family the researchers identified consists of five proteins. It remains inside the bacterial periplasm and is known as periplasmic cytochrome (Ppc)ABCDE. The proteins inject electrons into filaments on bacterial surfaces that act as nanowires, making an electric connection for metal breathing Geobacter.
This electrical connection allows Geobacter to transfer excess electrons produced during metabolism to minerals in soils, eliminating the need for oxygen-like soluble membrane-ingestible electron acceptors. Indeed, the proteins act as plugs that power the nanowires to create a type of natural “electrical grid” in the soil. The study was co-led by Nikhil Malvankar from Yale University, USA, and Carlos Salgueiro from NOVA University Lisbon, Portugal. According to the HFSP Research Grantees, this grid may be responsible for allowing many types of microbes to survive and support life.
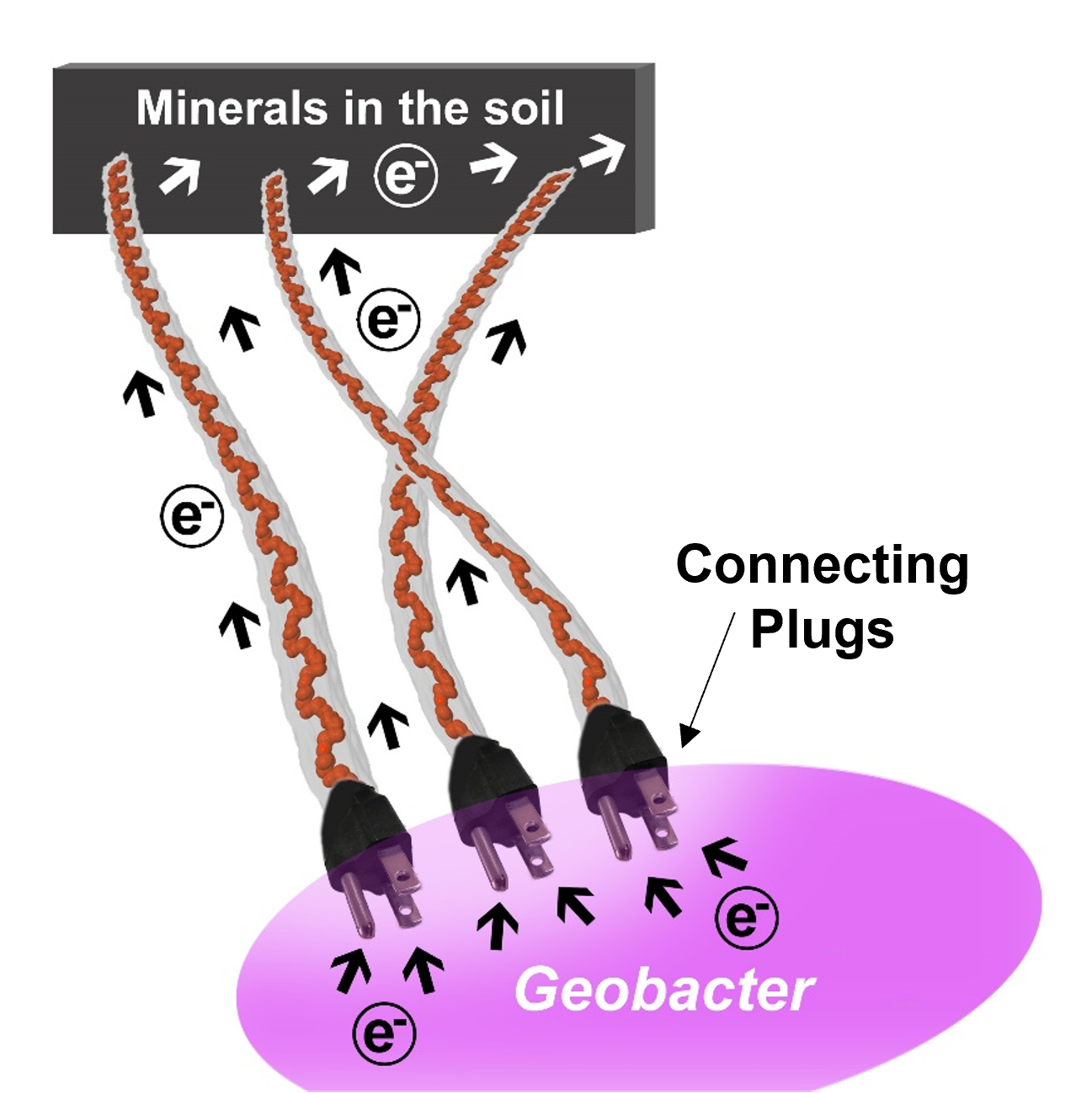
Pistons to push filaments made up of cytochromes
The filaments on bacteria were first observed in 2002, but until recently, they were thought to be made up of pili proteins (pili means hairs in Latin). This was because many bacteria have pili on their surface and all the genetic data suggested a similar role in Geobacter. In 2021, Malvankar’s lab solved the atomic structure of pili and showed that they instead act as pistons to push filaments made up of cytochromes. The atomic structures of the cytochromes known as OmcS and OmcZ showed a chain of metal-containing heme molecules that carry electrons. While these atomic structures explained how nanowires transport electrons, how they connect these nanowires to their surface remained a mystery, mainly because most cell surfaces are electrically non-conducting.
The answer was porin cytochromes, another family of proteins embedded in the bacterial membrane responsible for this connection despite bacteria being able to transmit electricity even in their absence. Nikhil Malvankar explained that “the presence of periplasmic proteins transferring electrons to nanowires eliminates the need for any intermediate electron carriers and explains how cells transmit electrons at a remarkably fast rate (100 million electrons per second), even though electrons in proteins can move at rates at least 1000 times slower.”.
PpcA-E could directly donate electrons to OmcS
The HFSP researchers began their experiments by measuring the electron energy in OmcS and found that it was the same as in PpcA-E. In 2015, Salgueiro’s group have hypothesised that PpcA-E’s could transfer electrons to OmcS. However, testing this hypothesis at that time was not feasible due to the limitation in obtaining purified OmcS nanowires. Later, the research group suggested that all PpcA-Es can couple electrons and protons, a prerequisite for generating energy which led them to propose that PpcA-E could pass electrons to nanowires.
The HFSP Research Grant enabled the researchers to confirm their hypothesis using Nuclear Magnetic Resonance (NMR) spectroscopy measurements. The Grantees team is now engineering the newly discovered mechanism into other bacteria that are important for the climate or that are capable of making biofuels to make them grow faster.


































