Biofilms, which are communities of bacteria embedded in a polymer matrix, are a major cause of chronic infection, morbidity, and death in many patient populations, such as those with cystic fibrosis, diabetes, or implanted medical devices. Consequently they result in huge healthcare costs and a poor quality of life for many patients. Bacterial cells growing in biofilms are difficult to treat and are protected from antibiotics and the immune system. Consequently, biofilms contribute hugely to the problem of antibiotic resistance, which is one of the key challenges of our time. In addition to the medical problems they cause, biofilms also damage built infrastructure by causing bio-corrosion and reduce the efficiency of water treatment and shipping by causing bio-fouling. On a more fundamental level, biofilms are an experimentally-tractable model for multicellular organization, cooperation, and competition. As a result of their ubiquity and impact, biofilms have been widely studied and a standard paradigm for the “biofilm life-cycle” has been developed. In this paradigm, biofilms initiate when free-swimming, so-called “planktonic” bacteria attach to a surface and begin to modify their gene expression profiles and to proliferate on the surface, eventually developing into a three-dimensional, multicellular structure.

Figure: The presence of multicellular aggregates at the start of biofilm growth is reflected in the structure of the biofilm a day later. Shown are perspective projections created from confocal microscope z-stacks of Pseudomonas aeruginosa biofilms. (A) Single cells attached to the surface at 0 h. (B) A preformed aggregate surrounded by single cells on the surface at 0 h.
(C) Biofilm descending from single cells from panel A. (D) After 24 h of growth, a large biofilm structure descending from the preformed aggregate shown in panel B surrounded by biofilm descending from single cells. Figure first appeared in MBio. 2016 Mar 22;7(2). pii: e00237-16. doi: 10.1128/mBio.00237-16.
However, biofilms can also initiate when pre-existing aggregates of bacteria attach to a surface. Such aggregates are known to arise spontaneously in liquid cultures of bacteria and to be shed from mature biofilms. Yet, the role played by bacterial aggregates in biofilm initiation has hitherto been neglected, even though aggregates have many traits commonly associated with biofilms, including increased resistance to antibiotics, cell-to-cell signaling (quorum sensing), and a three-dimensional structure that is at least 10 times greater in size than a single bacterium. In two linked papers, we examine how the spatial characteristics of pre-existing, multicellular bacterial aggregates impact their contribution to the growth of biofilms.
In a paper recently published in PLoS ONE, we use agent-based computational modeling to show that the degree to which aggregates contribute to the biofilm’s accumulated biomass is strongly dependent on both the aggregate shape and on the amount of competition for growth resource arising from single cells that co-seed the biofilm along with the aggregate. If the density of single cells, and therefore the amount of competition, is low, then aggregates that are more spread out, so that they are flatter on the surface, are more successful than rounded, more-circular aggregates. This arises from the larger surface area of the flatter aggregates, which allows more access to diffusing growth resources. However, if the density of single-cell competitors is high, then aggregates that are more rounded, so that their top cells are higher above the surface, are more successful than spread-out aggregates that do not extend as far off the surface. This arises because cells at the top of the aggregate have better access to growth resources than cells on or near the surface, where the high level of competition results in resource depletion.
These themes of competition and shape carry forward in a second recent publication in mBio that combines computational modeling with in vitro experiments. We show using both experiments and computer simulations that aggregates can have a competition-dependent growth advantage over single bacterial cells during the early stages of biofilm development. When the seeding density of single cells, and therefore the amount of competition for growth resource, is high, aggregates are fitter than single cells – we measure fitness as the ratio of accumulated biomass to initial biomass. However, when the seeding density of single cells is low, and so is the competition, single cells are fitter than aggregates. The fitness of single cells greatly decreases upon going from low competition to high competition, while the fitness of aggregates remains nearly unchanged. At low competition, aggregates have an overall disadvantage, compared to single cells, because the aggregate interior has very limited access to growth resource and grows slowly, while single cells on the surface have good access to growth substrate. In contrast, at high competition, aggregates have an overall advantage, because cells at the top have unfettered access to growth substrate that is almost entirely depleted at the surface. Slow-growing cells in the bottom and interior of the aggregate pay a cost so that cells at the top benefit.
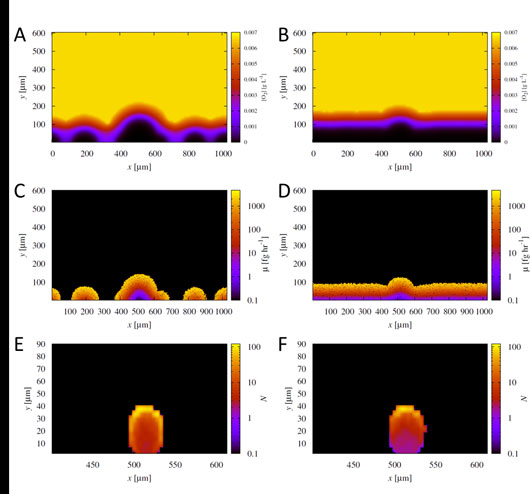
Figure: Biofilm morphology shapes the oxygen concentration profile, with the fittest cells initially located at the top. (A and B) The oxygen concentration (in grams liter−1) (right-hand y axes) in a sample simulation after 30 h of growth of cells at low density (0.01 cell µm−1) (A) and high density (0.5 cell µm−1) (B). x and y (both in micrometers) are the spatial dimensions of the simulation domain. (C and D) Growth rate (μ) (right-hand y axes) for the resulting populations after 30 h of growth at low density (0.01 cell µm−1) (C) and high density (0.5 cell µm−1) (D). (E and F) 2D histograms representing the number of progeny,N (right-hand y axes), produced after 30 h of growth by individual bacteria as a function of their initial location in the aggregate: low density (0.01 cell µm−1) (E) and high density (0.5 cell µm−1) (F). These distributions were averaged over 40 simulations for each aggregate. Note that the gradient in the number of progeny is so large that a log scale is used for visualization purposes. Figure first appeared in MBio. 2016 Mar 22;7(2). pii: e00237-16. doi: 10.1128/mBio.00237-16.
Taken together, these two papers show that a hitherto neglected aspect of biofilm development, namely initiation from pre-existing aggregates, can contribute disproportionately to the accumulation of biomass in the biofilm in a way that depends both on the level of competition and the shape of the aggregate. This suggests a new model for the biofilm life-cycle that accounts separately for contributions from single cells and aggregates. In future, such a model could be expanded to take into account additional fitness advantages for aggregates, such as resistance to antibiotics and to phagocytic immune cells. Interestingly, the formation of cellular aggregates has recently started to be discussed as a possible first step in the evolution of multicellularity. We hope to explore this idea in our future work.
References
[1] Shaping the Growth Behaviour of Biofilms Initiated from Bacterial Aggregates. G. Melaugh, J. Hutchison, K.N. Kragh, Y. Irie, A. Roberts, T. Bjarnsholt, S.P. Diggle, V.D. Gordon, R.J. Allen. PLoS One. 2016; 11(3): e0149683. doi: 10.1371/journal.pone.0149683.
[2] Role of Multicellular Aggregates in Biofilm Formation. K.N. Kragh, J.B. Hutchison, G. Melaugh, C. Rodesney, A.E. Roberts, Y. Irie, P.Ø. Jensen, S.P. Diggle, R.J. Allen, V. Gordon, T. Bjarnsholt. MBio. 2016 Mar 22;7(2). pii: e00237-16. doi: 10.1128/mBio.00237-16.


































