Over 100 trillion synapses exist in the human brain. These synaptic connections process input and store information for as long as lifetime. However, the molecular ‘substrates’ of memory, the proteins, only have one week's average ‘shelf-life’. This means synapses must constantly degrade old or damaged proteins and produce new copies to replace them. The ‘renewal’ of proteins is key to the homeostasis of brain function. And its dysfunction leads to neurological disorders. Most cellular proteins are degraded by a multi-component protein nanomachine called the proteasome.
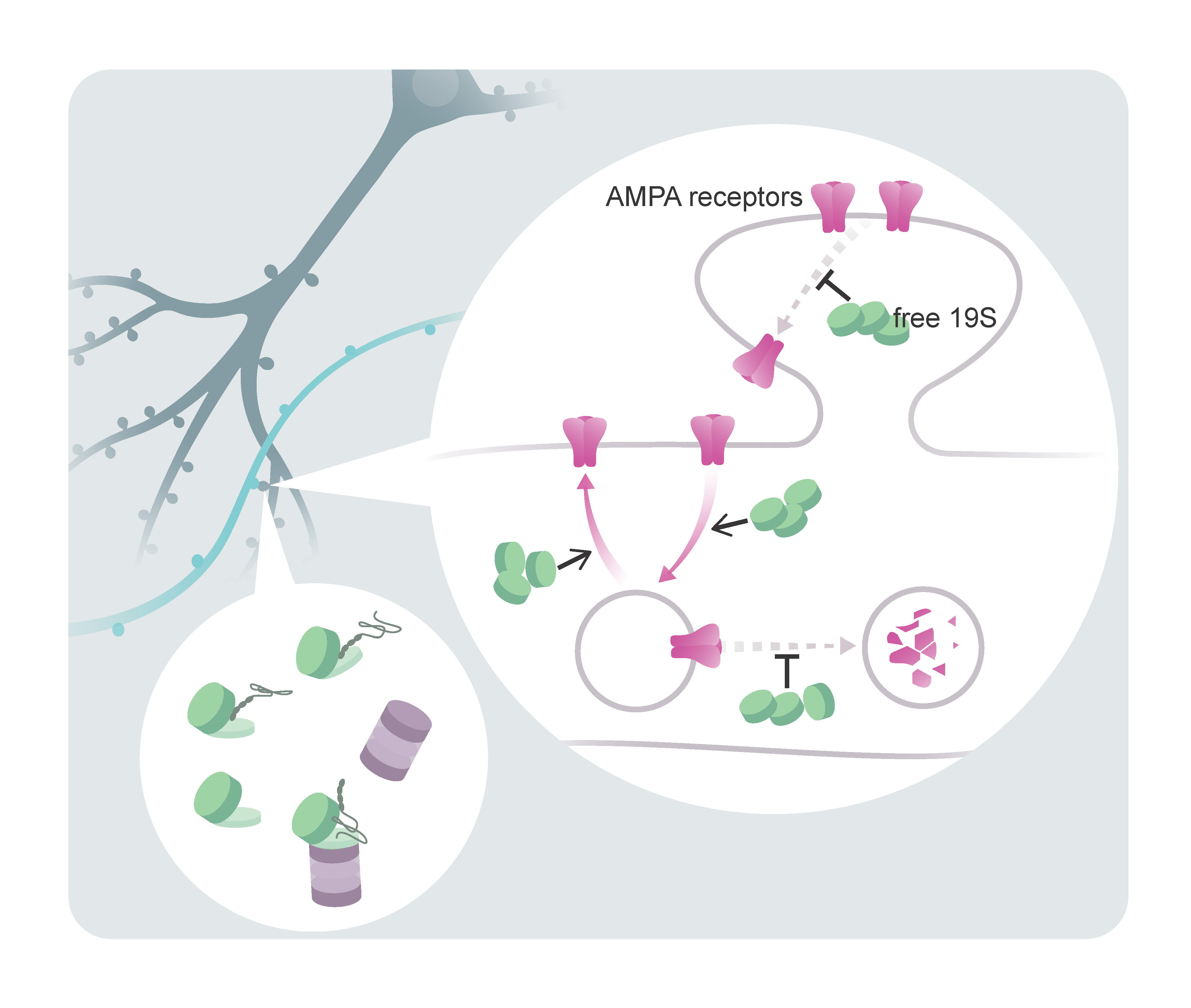
The proteasome (the cell’s protein garbage-disposal system) has two components: one for recognizing the to-be-disposed proteins (the so-called 19S complex) and the other for breaking them down (the 20S complex). Until recently, it was thought that the 19S only worked with its partner, the 20S complex. However, the HFSP cross-disciplinary fellow Chao Sun recently published a study in Science, showing that this is not always the case.
In the remote regions of a neuron- the dendrites, where information is transferred from one neuron to another at synapses- “super-resolution” visualization methods showed that the 19S complex of the proteasome is much more abundant than the 20S and can often be found alone. Surprisingly, functional studies showed that the free 19S particle regulates synaptic proteins without promoting their degradation. It recognizes a special group of synaptic molecules (e.g., neurotransmitter receptors, also called AMPA receptors) and changes their dynamics and localization within the synapse. This study reveals an intriguing and flexible aspect of synapse biology, where complex protein machines have likely adapted to subcellular needs and take on alternative functions.
Chao Sun did his postdoc with Erin Schuman, Director of the Max Planck Institute for Brain Research. The HFSP cross-disciplinary fellowship was crucial for enabling Chao Sun’s field switch from chemistry to neuroscience during his postdoctoral training and supporting his experiments that led to this discovery. The HFSP Awardee is now an independent group leader at the Danish Research Institute of Translational Neuroscience – DANDRITE (Nordic-EMBL Partnership for Molecular Medicine) and Aarhus University, Denmark.


































