During mitosis, the mitotic spindle partitions replicated chromosomes into two daughter cells. The spindle assembly checkpoint delays mitotic progression in cells in which not all chromosomes are properly attached to microtubules of the mitotic spindle. This ensures that cells have enough time to correct any attachment errors, allowing chromosomes to be properly segregated into daughter cells. Interestingly, cells exhibit a large variation in the extent to which they delay mitotic progression upon activation of the checkpoint. This is particularly evident during early embryogenesis of different metazoans: some embryos, such as those of X. laevis or D. rerio, display no SAC response during early embryonic divisions; other embryonic cells, such as those of C. elegans, exhibit only moderate mitotic delays; and others, such as those of M. musculus, display strong checkpoint responses from the start of embryogenesis. The absence of mitotic checkpoint signaling in some early embryonic divisions has been attributed to a developmental timer that only switches on checkpoint signaling at later stages of development, often around the onset of gastrulation. Another popular hypothesis is that the large size of many newly fertilized embryos results in dilution of the chromosome-generated checkpoint signal, resulting in weak checkpoint strengths. However, there was currently no clear evidence in vivo to support this hypothesis.
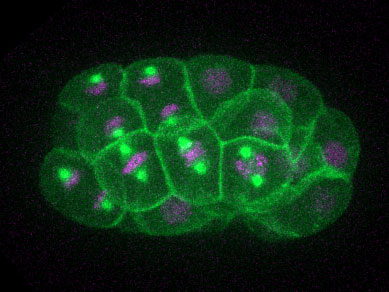
Figure: A C. elegans embryo undergoing divisions. The embryo expresses a membrane marker (GFP-PH in green), a microtubule marker (GFP-TBA-2 in green) and a marker for the DNA (mCherry-HIS-58 in magenta).
To determine what the factors are that control mitotic checkpoint strength during embryogenesis, we systematically analyzed checkpoint strength during early embryogenesis in C. elegans. For this, we used live microscopy to determine the mitotic delay induced by microtubule disruption at different stages of embryonic development. Strikingly, we found a gradual increase in the arrest time, ranging from the reported 2-2.5-fold delay in two-cell stage embryos to a 10 fold delay at the 50 cell stage. We hypothesized that the gradual increase in strength of the checkpoint could be explained by a decrease in cellular size in older embryos. Specifically, the strength of the mitotic checkpoint could depend on the ratio between the amount of unattached kinetochores, where the checkpoint signal is generated, and the amount of cytoplasm. To test whether cell size, and not a developmental timer, determines the strength of the checkpoint, we induced a spread in embryo sizes by depleting ani-2, and then assayed the mitotic delay in various sized two-cell stage embryos. Our results showed that the smaller cells are, the longer the mitotic delay is, arguing against a developmental timer controlling the strength of the checkpoint. Furthermore, we found that triploid embryos, which have an increased amount of kinetochores, exhibit a stronger mitotic delay upon disruption of the mitotic spindle. Together, these results demonstrate that the kinetochore-to-cytoplasm ratio determines the extent to which embryonic cells delay mitosis upon activation of the spindle assembly checkpoint.
To understand how cell size controls the strength of the mitotic checkpoint, we looked at the effectors of checkpoint signaling. During checkpoint signaling, unattached kinetochores recruit a tight complex of the checkpoint proteins Mad1 and Mad2, which generates a catalytic platform for the production of a mitotic checkpoint complex. This checkpoint complex then directly binds and inhibits the APC/C, an E3-ubiquitin ligase that promotes mitotic exit. We looked at concentrations of Mad1, Mad2 and APC/C during different stages of embryogenesis and found that concentrations remain constant as cells become smaller. Because the relative amount of kinetochore increases in smaller cells, our results suggest that the relative amount of inhibited APC/C, and not the absolute amount of checkpoint proteins or APC/C, determines checkpoint strength in embryonic cells. Together, these findings provide new insights into why cells exhibit large variations in their ability to delay mitotic progression upon disruption of the mitotic spindle.
Reference
Cell Size Determines the Strength of the Spindle Assembly Checkpoint during Embryonic Development. Galli M, Morgan DO. Dev Cell. 2016 Feb 8;36(3):344-52.
Other references
Commentary on Dev Cell: Bigger Isn't Always Better: Cell Size and the Spindle Assembly Checkpoint. Gerhold AR, Labbé JC, Maddox PS. Dev Cell. 2016 Feb 8;36(3):244-6.


































