Morphogenesis embraces cellular processes that cause changes in tissue shape, critical for the correct functioning of organs. Although it is regulated by signaling pathways that direct cell proliferation, fate and transport, morphogenetic processes shaping the form of tissues ultimately rely on force-generating events that arise from collective cellular behaviors. Unveiling the cues that direct such behaviors is fundamental not only in gaining understanding on how tissues are formed and deformed, but also to open the door to new design principles for tissue engineering and regenerative medicine.
One of the cues controlling how cellular activity is dissipated into steered motion is orientation. In tissues composed of cells with elongated morphologies, cell-cell interactions may promote multicellular alignment, leading to the formation of large domains featuring orientational order. Regions in between these ordered domains, where the order is ill-defined, configure the so-called topological defects (Figure 1). On one hand, orientational order imposes easy flow directions for cell migration in tissues. On the other hand, the presence of topological defects in these active materials implies very strong gradients in orientation that lead to reproducible and robust flow and force patterns that will depend on the type of defect and its topological charge(s).
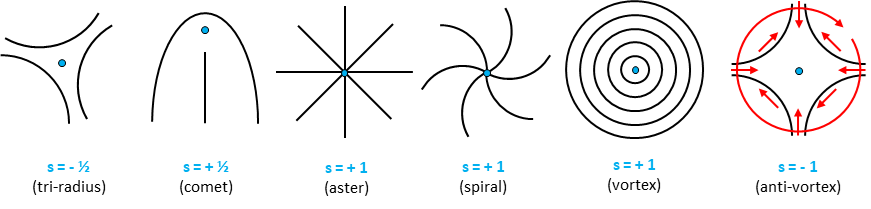
Figure 1: Topological defects. Black lines depict orientation around typical topological defects in 2D. Blue dots indicate the position of the defects’ core. Topological charge(s) and common name of the defects are indicated below. Red arrows represent the rotation of a vector around the defect with s=-1. Note that a clockwise rotation around the defect’s core implies a full counterclockwise rotation of the vector.
HFSP Cross-Disciplinary Fellow Pau Guillamat, in the laboratory of Aurélien Roux (former HFSP fellow and HFSP Career Development Award holder), together with theoretical colleagues in the University of Geneva (Switzerland), studied the dynamics, mechanics and biological role of specifically two types of cellular topological defects: spirals and asters (Figure 2A). To achieve this, the authors confined nematic myoblast monolayers into cell-adhesive circular disks coated with the extracellular matrix (ECM) protein fibronectin and surrounded by a cell-repellent polymeric brush (Figure 2B). Importantly, the circular geometry requires the presence of at least one topological defect. Indeed, when confinement size was smaller than a critical size, related to the orientational order length scale, cells were forced to self-organize into spirals or asters.
By combining experiments and theory (see other references, below), this work explores the dynamics and mechanics of these active arrangements, which provide, through unique organizational cues, cellular activity to be harnessed into specific flow and force fields. The authors show how the presence of these defects is sufficient for organizing tissue remodeling events such as differentiation and 3D tissue growth. These very same cues could be involved in directing active stresses within tissues during morphogenetic processes in vivo during development and disease.
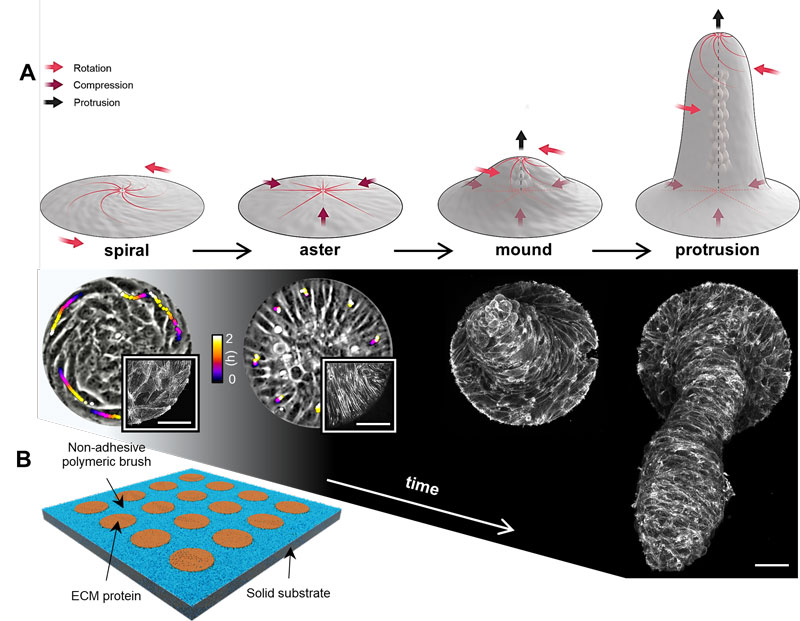
Figure 2: Spiral and aster defects direct the growth and dynamics of multicellular protrusions. A, Time evolution of myoblast islands and implied forces (top illustration by Margot Riggi). Bottom layout shows phase contrast and confocal micrographs of the different multicellular arrangements. In spirals and asters, color coded lines indicate single cell trajectories. Insets correspond to actin-labelled spirals and asters. The mound and the protrusion are shown as maximum z-projections of actin. Scale bars, 50µm. B, Schema of the experimental setup.
|
HFSP award information Cross-Disciplinary Fellowship (LT000793/2018-C): In vitro control of cell collective flows and tissue folding by means of surface patterns Fellow: Pau Guillamat |


































