Metastatic breast cancer (MBC) remains a leading cause of cancer-related deaths, affecting countless women worldwide despite advances in targeted therapies and immunotherapies. Scientists now recognize that the diversity of cells within tumors and their interactions with surrounding tissues—the tumor microenvironment (TME)—play vital roles in cancer progression. To better understand MBC biology, HFSP researchers have developed novel profiling methods to study the unique cellular and molecular landscapes in metastatic tumors.
In a recent study, Johanna Klughammer, HFSP Fellowship Awardee at the Broad Institute of MIT and Harvard and her team undertook a comprehensive mapping of 67 metastatic breast cancer biopsies from 60 patients using advanced single-cell and spatial expression profiling techniques. These methods allowed for a comprehensive view of each biopsy, covering a range of clinicopathological characteristics, tumor locations, and receptor configurations. By analyzing individual cells and their spatial organization, the team provided fresh insights into how different profiling methods represent MBC biology and how these diverse data types can be integrated to reveal new patterns of tumor progression.
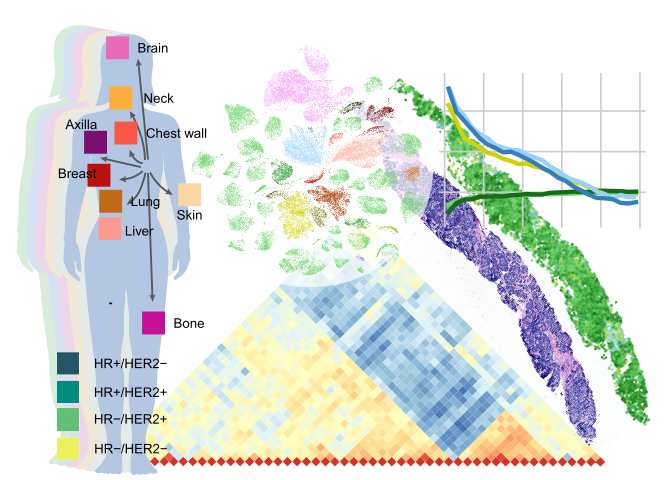
The study demonstrated the strengths and limitations of four distinct spatial expression profiling techniques, each offering unique insights based on their resolution and focus. For example, MERFISH enabled the identification of immune cell exclusion zones near certain cancer cells, while scRNAseq data indicated that malignant cell expression profiles remain unexpectedly stable over time within individual patients. These findings underscore the need to consider spatial organization and stable expression patterns in developing future MBC therapies, potentially leading to more effective treatments and improved patient outcomes.
Overall, this work is a technical milestone in MBC research, showing how detailed maps of metastatic tumors can lead to clinically relevant insights. While the study’s broad focus highlighted general trends rather than specific clinical questions, it opens avenues for future research aimed at understanding MBC’s complexity. The dataset generated in this study is expected to serve as a valuable resource, guiding the next generation of cancer studies toward more effective treatments and, ultimately, better patient outcomes.


































