Cells in our body are constantly exposed to changes in their environment. When tissues are injured, surrounding cells must quickly move to close gaps and restore the protective barrier. This process of wound closure is not random. Instead, cells adopt different strategies for migration depending on the shape of the wound. They extend lamellipodia, which are flat membrane protrusions, to crawl forward at outward curving (convex) edges, while they assemble a contractile actin ring that tightens like a purse string to pull the edges together at the inward curving (concave) edges. What has remained unclear is how cells sense their local environment and the wound’s shape, and how they decide which of these modes of movement to use.
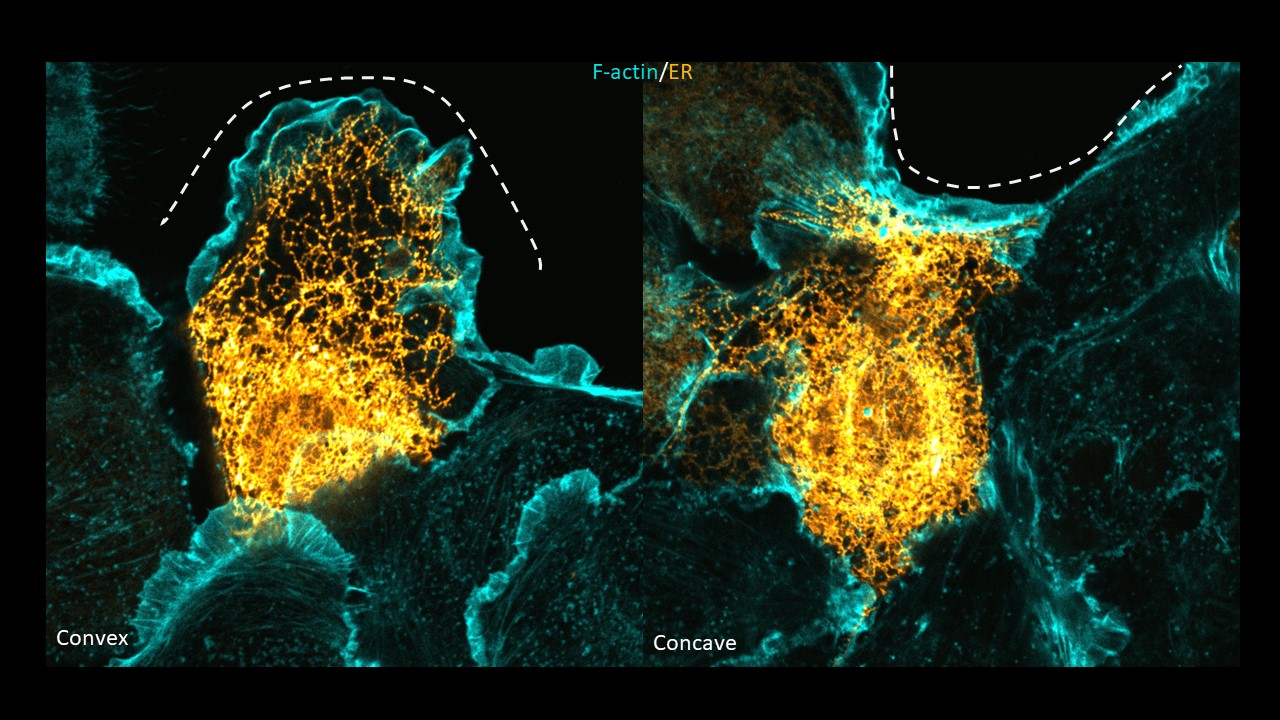
In the current study, published in Nature Cell Biology, the HFSP Research Grant Awardee Tamal Das and his colleagues describe an intracellular cartography of cytoskeletal proteins and organelles in response to different wound shapes. This study identifies the endoplasmic reticulum (ER) as a central player in sensing and responding to the wound curvature. The ER, a large and dynamic membrane network, is traditionally viewed as a site for protein and lipid synthesis. However, the team discovered that its shape is not static. Instead, it reorganizes according to the geometry of the wound edge. At outward-curving (convex) edges, where cells usually form lamellipodia to move, the ER forms thin tubules and at inward-curving (concave) edges, the ER spreads into broad sheets that associate with actin-driven purse-string contraction. Das and his colleagues altered the ER morphology using expression of ER-morphology deciding proteins, and interestingly observed that increasing ER tubules in the cells led to cells forming lamellipodial membrane extensions at both curvatures. They conclude that this distinct difference in the ER morphology plays a crucial role in deciding the mode of cell migration.
To understand the physical mechanism of why a cell would choose to modify its intracellular organization in response to wound shape, the researchers developed a mathematical model in collaboration with Pradeep Keshavanarayana and Fabian Spill at the University of Birmingham. The model shows that while migrating at different curvatures, the cells experience minimum strain energy if ER is tubular at convex edge and if it is sheet-like at the concave edge. By linking curvature to organelle structure and then to cell behavior, these findings show that the ER is not simply a background component of the cell but an active sensor and regulator. It translates geometric information into functional changes that determine how a tissue moves and repairs itself. This perspective shifts the way we think about organelles: rather than being confined to internal housekeeping functions, they can have direct roles in collective cell responses at the tissue level. The broader implications of this study are significant. Collective migration underlies processes ranging from wound healing to cancer invasion and embryonic development. Understanding how the ER biases migration modes provides a new entry point for studying these events in both normal and disease settings. It also raises intriguing possibilities that manipulating ER organization could help guide tissue repair or control invasive behaviors in cancer.
Overall, the work uncovers a surprising and important role for the ER in coordinating cellular behavior during tissue remodeling. It underscores how cells integrate physical cues from their environment with internal organelle dynamics to make decisions that ultimately maintain tissue integrity.


































