To get life-giving oxygen into every cell, the human body produces two to three million oxygen-carrying red blood cells, or erythrocytes, each second – about one quarter of all the new cells getting produced in the body at any one time. While erythropoiesis mainly takes place in the bone marrow, it’s key regulator hormone erythropoietin (Epo) is produced in the kidney. The kidney thus acts as a central sensor of systemic oxygen supply. While excessive Epo leads to erythrocytosis and consequently thrombosis, hypertension, and death, too little Epo results in anemia. Despite their physiological importance, the identity of which kidney cells produce Epo remained unknown – until now!
It all started with using the paradigm of Tibetans living in high altitude hypoxia. How have Tibetans increased their fitness to live in a low oxygen environment whereby erythropoiesis normally would be overstimulated leading to erythrocytosis and decreased fitness? Over hundreds of generations, the Tibetan genome has evolved protective variations that enable reduced erythrocytosis-related mortality and increased birth rates compared to recent migrations of Han-Chinese to the Tibetan plateau.
If the Epo producing cells, similarly to the insulin producing cells on the pancreas, are so important, why weren’t the cells identified so far? What could be done differently? What was already known? The cells were known to be notoriously difficult to isolate and the only known marker was Epo itself. The cells produce very little Epo in normal oxygen conditions and exhibit irregular Epo production during hypoxia.
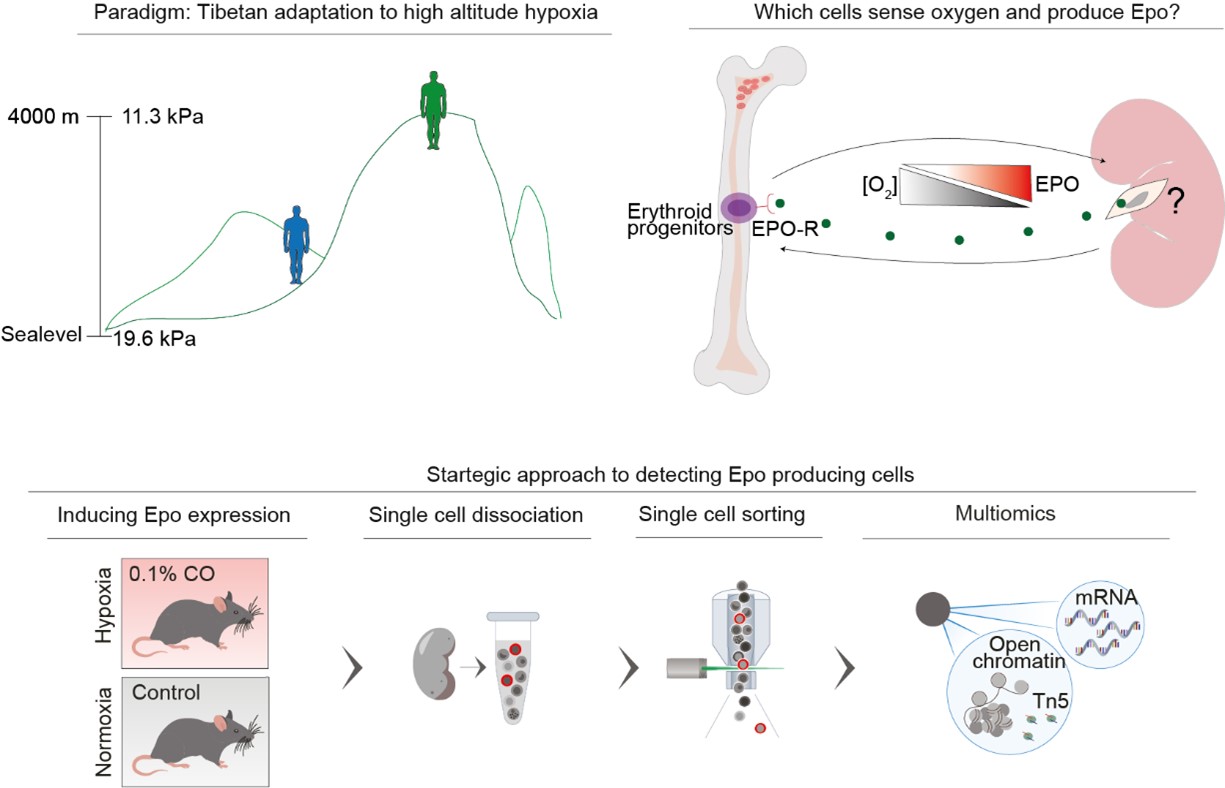
Bjørt K. Kragesteen was awarded a Postdoctoral Fellowship from HFSP [LT000230/2019-L] to take on a novel approach to that: combine human evolutionary studies with cutting edge single cell technologies. For this purpose the HFSP Fellowship Awardee joined the laboratory of Prof. Ido Amit at the Weizmann Institute of Science (Israel), to use single cell analysis methodologies that enable the study of thousands of individual cells simultaneously and thus the identification of rare types of cells in tissues.
With the targeted defined, the study successfully uncovered that Epo production is restricted to a single, unique, and evolutionary conserved population of kidney fibroblast-like cells, which were named Norn cells, after the mythological Norse creatures believed to spin the threads of fate. The analysis uncovered thousands of Norn regulatory regions in the genome as well as transcription factor candidates that regulate these regions in the cells.
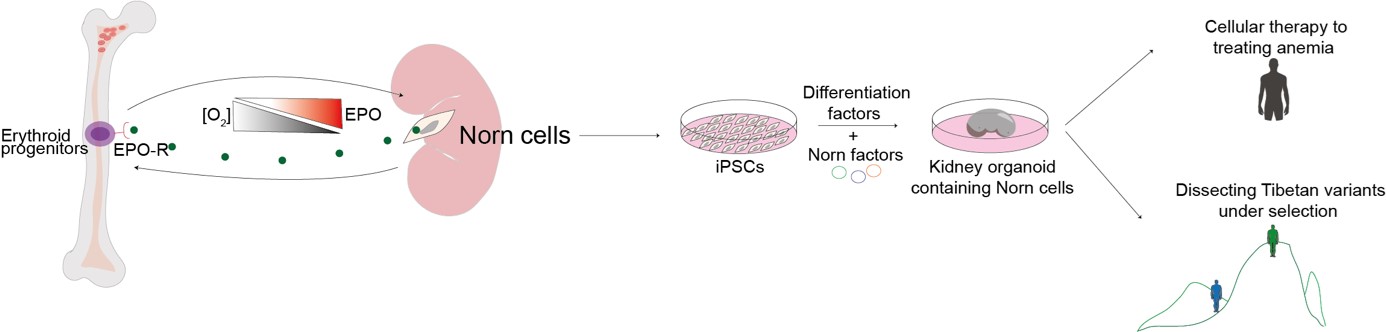
The discovery has transformative potential, both to dissecting the functionality of variants identified in Tibetan population and to generate Norn cells in vitro using the newly identified transcription factors. Moreover, revealing the Epo-making cells is vital, for the 10% of the population that develop chronic kidney disease and resulting anemia and relying on recombinant Epo injections. The researchers hope that the discovery of the Norn cells may thus shed new light on Norn cell regulation to develop better therapies as well as cellular therapies.


































