Emotion plays a crucial part in our lives, regulating our actions, our responses to the environment and our ability to form memories. Emotion helps us identify meaningful events, avoid potentially dangerous situations and form richer, more lasting memories of events that are potentially meaningful to us. However, the negative emotions that are associated with fearful memories can cross a certain threshold and become maladaptive — as occurs in anxiety disorders and post-traumatic stress disorder (PTSD). Therapeutic approaches for fear and anxiety disorders are often ineffective, partly due to an incomplete understanding of the brain circuits that regulate these processes. Although fear memories can be extinguished through repeated exposure to the previously-threatening situation under a “safe” environment, knowledge is still limited regarding the brain circuit mechanisms that mediate this process. In a study recently published in Nature Neuroscience, a team led by HFSP awardee Dr. Ofer Yizhar of the Department of Neurobiology at the Weizmann Institute of Science, demonstrated that it is possible to reduce the acquisition of “cued” responses to frightening memories. To achieve this goal, the team used a technique called optogenetics—a technology that renders individual, highly specific brain cells photosensitive and then activates those cells using flashes of light.
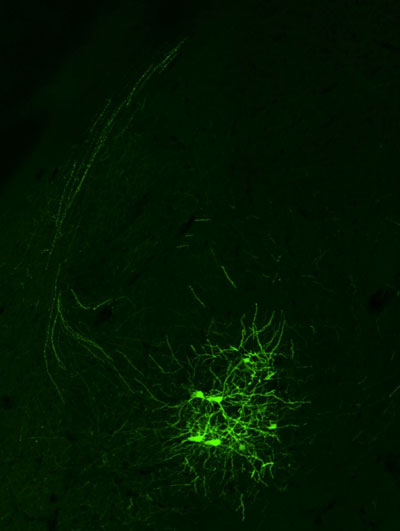
Figure: An example of neurons in the mouse amygdala, labeled based on their projections to the prefrontal cortex.
In the team’s experiments, mice were exposed to fear-inducing conditions in parallel with a sound that the mice learned to associate with fear. Mice then displayed fear responses when hearing this tone, much in the same way that Pavlov’s dogs had learned to expect food upon hearing a bell ring. After mice acquired this fear-inducing memory, the researchers used laser light to “tune” the strength of neural connections between the amygdala, a region involved in emotional processing, and the prefrontal cortex – a region known to be crucial for higher cognitive function. Using engineered viruses, they expressed a gene coding for the light-gated ion channel called “channelrhodopsin” in the amygdala. Expression of channelrhodopsin allows scientists to stimulate the neurons, or their projections throughout the brain, simply by directing blue light to these cells through a tiny optical fiber.
The team found that high-frequency light-mediated activation of the synaptic connections linking the amygdala and the prefrontal cortex caused a reduction in the strength of neurotransmission along this pathway. When this stimulation method was used in mice that had acquired a fear association, the mice responded more rapidly to a “fear extinction” protocol, displaying less and less fear as time went by, leading to more efficient extinction of the fear-associated memory than in control mice that had not undergone the optogenetic manipulation. The process through which mice learned to stop fearing a previously-fearful tone is thought to be similar to how some human PTSD sufferers eventually become resilient in the presence of a stimulus that once triggered a trauma sensation. By clarifying how signals that bridge two areas of the mammalian brain contribute to the fear response, the team’s findings have shed light on the contribution of this pathway to the learning and extinction of fear-related associations. While much work still needs to be done in order to obtain a complete understanding of the circuits mediating fear and anxiety, optogenetic technology is helping researchers delineate the contribution of individual circuit elements to these behavioral states. Better understanding of the functional roles of defined circuits will potentially aid in refining current therapeutic approaches and the development of new treatments.
This is one of the first projects we have started in the lab, and as such it benefitted greatly from the CDA that I received on my first year at the Weizmann Institute. The CDA allowed us to pursue an ambitious project that stemmed directly from my main postdoc research paper (which was itself funded by an HFSP postdoctoral fellowship).
Reference
Manipulating fear associations through optogenetic modulation of amygdala inputs to the prefrontal cortex. Klavir O.*, Prigge M.*, Sarel A., Paz R., Yizhar O. Nat Neurosci. 2017 Jun;20(6):836-844. doi: 10.1038/nn.4523.


































