HFSP awardees Stephen Michnick and Rohit Pappu have discovered a new mechanism by which membrane vesicles are made. These self-contained nanoparticles trap proteins, RNA and other molecules from outside living cells as nutrients or regulate the numbers of cell surface hormone receptors, such as those for insulin, to control the sensitivity of cells to hormones. These processes are essential for the normal functioning of our cells and disfunctions are implicated in several diseases, including cancers, heart disease and neuropsychiatric disorders. Finally, it is the mechanism by which nanoparticles encapsulating mRNA vaccines are taken up into cells.
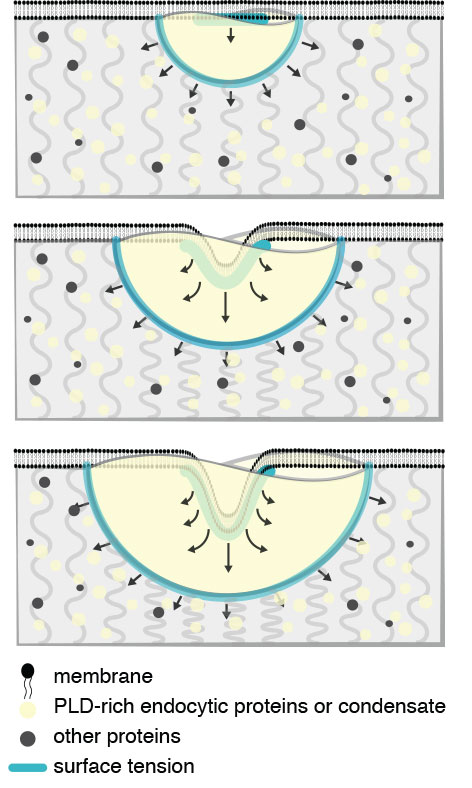 |
Figure Endocytic condensates perform mechanical work to deform the membrane and cytosol. Mechanical description of endocytic condensate-driven membrane remodeling in the absence of actin. Clathrin heavy and light chains (Chc1 and Clc1) interact with initiator proteins (Ede1 and Syp1) to form a lattice on the membrane. Subsequently, PLD-containing coat proteins (yellow), such as Sla1/2, Ent1/2, and Yap1801/2, directly bind to the initiator-clathrin lattice and form the endocytic condensate (yellow). The endocytic condensate (yellow) binds to (i.e., wets) the bilayer membrane (black) and drives membrane invagination as the condensate expands to maximize contact with the cytosol (top to bottom). Forces balance under a Young-Dupré adhesion gradient (blue lines and arrows) and resistance of the cytosol (grey curved lines). |
In a study published in the journal Proceedings of the National Academy of Sciences, the researchers discovered that endocytosis, the process by which molecules are transported into cells, begins by the formation of a ‘biomolecular condensate’. Recently discovered biomolecular condensates organise proteins and RNA into liquid droplets, reminiscent of oil droplets in water. In the initial stages of endocytosis, specific proteins coalesce at nucleation sites on the membrane of the cell, a bilayer structure of phospholipid molecules, like detergent molecules that form the soap-bubble-like structure of cell membranes. Over several seconds a condensate grows on the surface of the membrane, like dew on the surface of glass. At a critical moment the cell membrane buckles and invaginates into the condensate, ultimately being pinched off to form a spherical vesicle. How membrane buckling occurs has been a mystery, but the researchers thought that the endocytic condensate could be the key.
To understand how endocytosis could be initiated by a biomolecular condensate, lead author Louis-Philippe Sandoval-Bergeron reasoned that membrane buckling must have something to do with the material properties of the condensate. In collaboration with bioengineers Allen Ehrlicher and Adam Hendricks at McGill University, they used a method called Optical Trap Microrheology to determine the viscoelastic properties of the endocytic condensate. Then, together with theorist Paul François at McGill University, constructed a mathematical model to explain how the endocytic condensate forces the membrane to bend into the condensate to form a nascent vesicle (see Figure).
The study demonstrates that an emergent property of biomolecular condensates is to act as ‘mechanoactive devices’, able to work on other materials to shape and organize living cells. Much work lies ahead to confirm and extend the mechanoactive role of biomolecular condensates in other vesicle and membrane-bending processes that shape the cell and transport materials in and out. Finally, the proteins that make up the endocytic condensate are related to others that are implicated in neurodegenerative diseases and cancers. Mutations of these proteins change the material properties of condensates that they form and it is thought that these changes may be the cause of disease. Efforts are now underway to develop drugs that prevent or reverse these changes.
|
HFSP award information Research Grant - Program (RGP0034/2017): Elucidating the molecular logic of membrane-free compartment function and assembly Principal investigator: Stephen W. Michnick, University of Montreal, Canada |


































