The mechanism dosage compensation functions as a decisive factor for the dimorphic sex to be naturally selected and to evolve the sex chromosome by hyper-activating the single X chromosome in male Drosophila and maintaining equality in the diploid dosage in the same chromosome in females. A large plethora of transport proteins and transcription factors, that are autosomally located, are found to have targets that are sensitive to male aneuploid effects. There is a gradual shift of such targets from the primitive X-chromosome to autosomes in different recent species, which proposes that the transacting regulatory modifiers may counteract their targets, present on two different chromosomes, providing an evolutionary advantage. The binding of the MSL complex is strongly associated with numerous sites of the X chromosome, a requirement for the hyper-activation of the haploid male X chromosome.
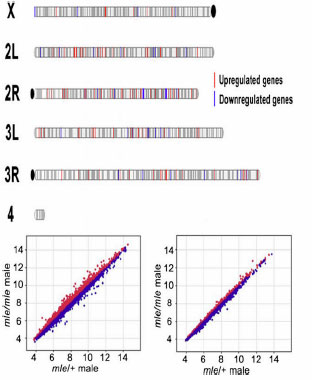
Figure: Loss of an entire X chromosome in male Drosophila produces a genome wide aneuploid effect. To resolve the aneuploid complexity, we generate a new function of the dosage compensation complex to execute additional workload that promotes compensation of inverse trans-acting aneuploid effect between aneuploid targets and X chromosomal modifiers together with other genes on the male X chromosome. The inverse interaction between the autosomal aneuploid target and X chromosomal modifiers is advantageous for the location of targets and modifiers in two different chromosomes. Genome wide distribution of aneuploid targets on 4 pairs of Drosophila chromosomes (top). The global pattern of gene expression of mle homozygous male (bottom).
A microarray analysis investigated the global aneuploid effect that a mutant maleless showed in creating a binding disruption of the male specific lethal protein of the MSL complex in compensating the dosage difference of the X linked genes. The complex, MSL, compensated the dose of the gene, firstly by hyper-activating the X-linked genes and afterwords compensated modifiers in male single copy, which in turn execute an inverse aneuploid effect to equalize the gene expression of the autosomal aneuploid targets in both sexes.
A genome wide screening using oligo-chips of DNA showed that the dosage of the gene was compensated only in the Drosophila soma. The analysis revealed a reduction in nearly 70% of the mle-dependent aneuploid-sensitive genes and showed a prominent reduction in the number of X-linked aneuploid regulators on the X chromosome relative to autosomes. The gene chip hybridization and the micro-array analysis data suggested that few of the MSL-targeted transcriptional modifier targets have shifted to the autosomes from the haploid X- chromosome. Thus proving that MSL performs multiple events, not just conditioning the X-chromosome compensation but it also maximizes the expression of the gene for both sexes. That variation in chromosomal damage is a catalyst to initiate an aneuploid effect is emphasized by the fact that multiple modifiers for aneuploidy can be restricted to the target genes in the same chromosome, which is in agreement with the principle of genomic balance.
This evolutionary strategy permits the migration to the autosomes. However, depending upon the manner in which the inverse aneuploid regulators link themselves to the aneuploid chromosome modifiers, the expression is maintained to normality. The consequence of such an activity is the binding of the MSL protein as a compensatory regulator for the aneuploid modifiers and targets in males. Hence, this work has highlighted the interactive mechanism which exists for the gene regulation across evolutions and compensation for the sexually dimorphic types.
The microarray profiling data of the Drosophila melanogaster presently reflects only 40% of the Anopheles genome while the other species of the ancient Drosophila family showed greater similarity. Thus, the aneuploid target shift is a very unique measure adopted by nature to positively favour the evolution of the environment. The Anopheles and the Drosophila might have been subjected to a shift of aneuploid target genes with a variable evolutionary distance.
Text by Utpal Bhadra and Paromita Das
For further information please contact Utpal Bhadra at Click here to show mail address
Reference
Drosophila maleless gene counteracts X global aneuploid effects in males. Bhadra U, Gandhi SG, Palaparthi R, Balyan MK, Pal-Bhadra M. FEBS J. 2016 Sep;283(18):3457-70. doi: 10.1111/febs.13818. PMID: 27456781.
Pubmed link


































