Bacteria are among the most ancient and successful forms of life, and their ability to navigate their environment to where their lives are better has been central to that success. Swimming allows bacteria to seek out food, avoid danger, and colonize new environments. By and large, this motion is powered by the bacterial flagellar motor, a rotating molecular machine that works much like a tiny propeller. Because flagella are found in so many species, biologists have long suspected that the earliest bacteria were already capable of swimming.
In this study, HFSP researchers confirmed that suspicion by conducting one of the most comprehensive surveys of bacterial motility to date. They analyzed over 11,000 bacterial genomes, searching for each of the 54 genes that make up the flagellar pathway. This large-scale comparison showed that the last common ancestor of bacteria had flagellar machinery, meaning that the ability to swim has been part of bacterial life since its very beginning. The team also found that motility has been lost far more often than gained throughout evolutionary history, highlighting that swimming, while powerful, is not always essential for survival.
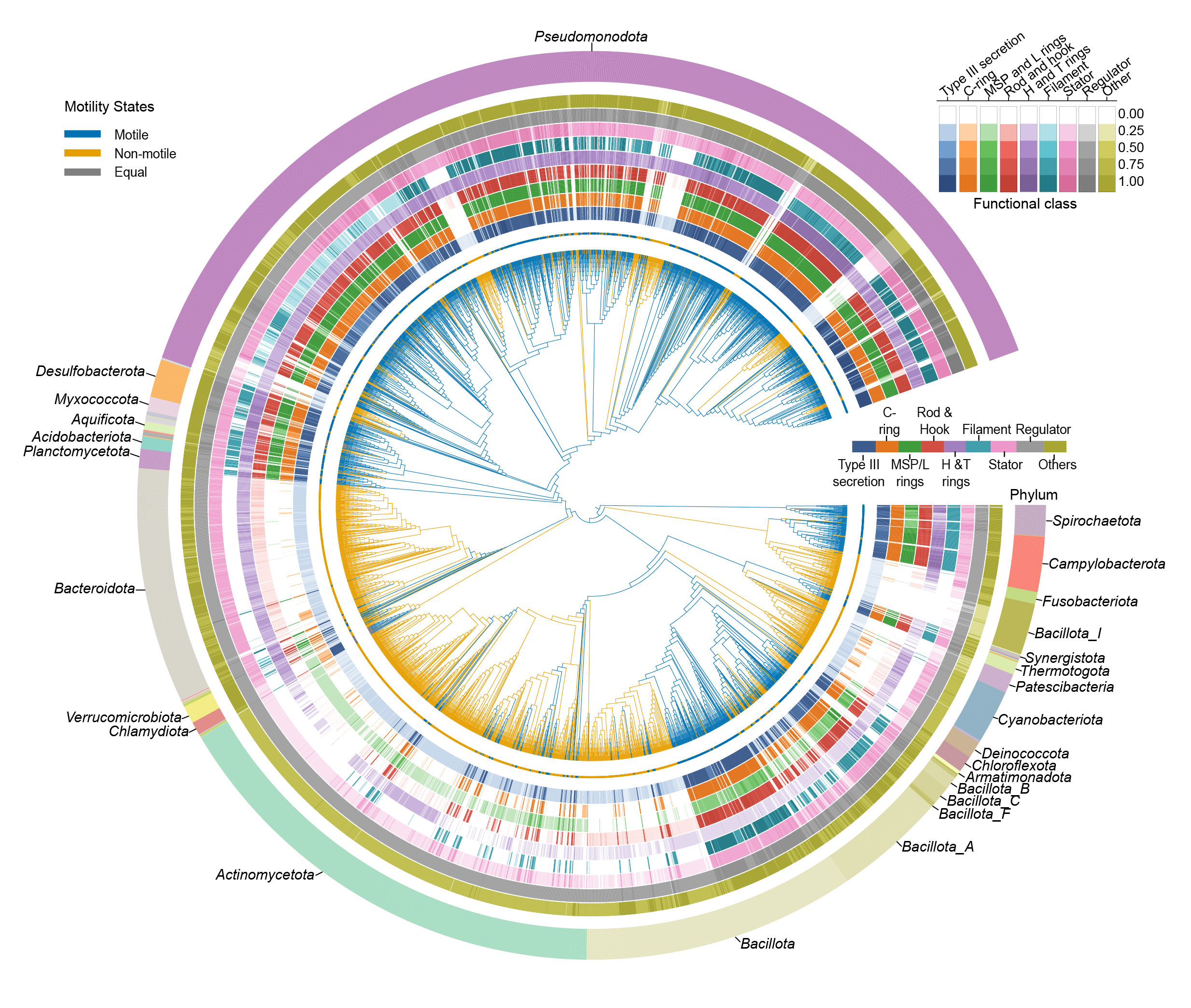
Beyond this broad evolutionary picture, the work led by Matthew Baker at the University of New South Wales, Australia, identifies the proteins most critical to motility. The study showed that the filament protein FliC, which forms the propeller itself, is the single most reliable predictor of whether a bacterium can swim. When species lose FliC, they invariably lose motility. Other parts of the machine, such as the rotor and hook, are also highly correlated with swimming, whereas more peripheral components are less conserved. Using these insights, scientists built a classifier that can predict with up to 95% accuracy whether a species is motile just from its genome. This provides a powerful new quick classification tool for microbiologists studying new types of bacteria in diverse environments.
This research project was supported by HFSP with a Research Grant that allows scientists from four countries - Australia, New Zealand, USA and UK - to collaborate and combine their expertise in trait analysis and microbial evolution with structural biology and phylogenetics.
Looking ahead, these findings open the door to practical applications: by predicting motility directly from genomes, researchers can better understand the ecological roles of microbes, how pathogens move through hosts, and even how motility might be engineered or disrupted in biotechnology and medicine.


































