Transcription is episodic, consisting of a series of discontinuous bursts. That is, multiple molecules of RNA polymerase II are released from the promoter consecutively followed by a refractory period. However, the underlying cause and biological significance of transcriptional bursting have been unclear.
In this study, we applied newly developed live-imaging methods to test the possibility that transcriptional bursting is caused by dynamic enhancer-promoter interaction. We visualized and analyzed the activities of several well-defined enhancers in living Drosophila embryos. We found a correlation between enhancer strength and the frequency of transcriptional bursting. Strong enhancers drive more frequent transcriptional bursting than weaker enhancers, thereby producing larger amount of mRNA molecules. When enhancer-promoter interaction was impeded by insertion of insulator DNA, we observed significant reduction in the frequency of transcriptional bursting and corresponding diminishment in gene expression. These results support the idea that enhancers control the frequency of transcriptional bursting to regulate spatiotemporal patterning of gene expression during development.
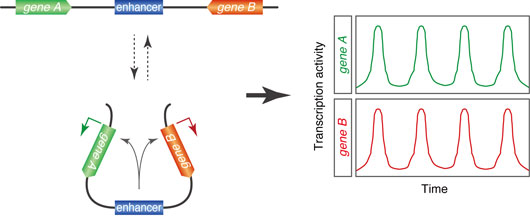
Figure1. Model for dynamic transitions in chromosome topology.
To further explore the dynamics of enhancer-promoter interactions, we developed a novel live-imaging method which permits simultaneous visualization of two different genes under the control of a shared enhancer. According to classical looping models, the shared enhancer is expected to interact with one of the linked promoters randomly to produce a burst and this should result in mutually exclusive expression of two target genes in a given nucleus. Surprisingly, however, we observed a high frequency of coordinated transcriptional bursting of the two linked genes, raising the possibility that an enhancer can simultaneously activate two promoters. Our observation suggests dynamic formation of a local DNA loop domain that positions two target promoters in close proximity with the single enhancer.
Reference
Enhancer control of transcriptional bursting. Takashi Fukaya, Bomyi Lim, Michael Levine. (2016) Cell 166 (2), 358–368.


































