The mitotic spindle is an out-of-equilibrium cellular machine that forms during cell division to segregate the genetic material in the two future daughter cells. Spindles are dense filamentous structures composed of microtubules, which are dynamic biopolymers that are continuously transported towards the two opposite ends - or poles - of the spindle. Such active transport is achieved by molecular motors, proteins that are capable of converting chemical energy into mechanical work. As a consequence, the motors generate a coherent poleward flow of microtubules within the structure known as the poleward flux. From a physical perspective, the mitotic spindle can be regarded as an active liquid crystal with unique material properties. One of its striking features is that microtubules are continuously created and disassembled on the order of seconds, while the structure can live for hours. How such a dynamic structure is capable of generating coherent flows is not understood. In addition, how large meiotic spindles acquire the proper polar arrangement of microtubules is also unclear. With support from HFSP, the authors recently showed that the size and mass of large spindles is controlled by branching microtubule nucleation [Decker 2018, Rieckhoff 2020], a process in which microtubule filaments nucleate from pre-existing microtubules, like branches in a tree. Microtubules are nucleated at the vicinity of chromosomes and branch outwards, yet in spindles microtubules point inwards to interact with chromosomes during segregation. The known mechanism of microtubule nucleation and the measured spindle architecture and microtubule flow profiles all stood in contradiction to each other.
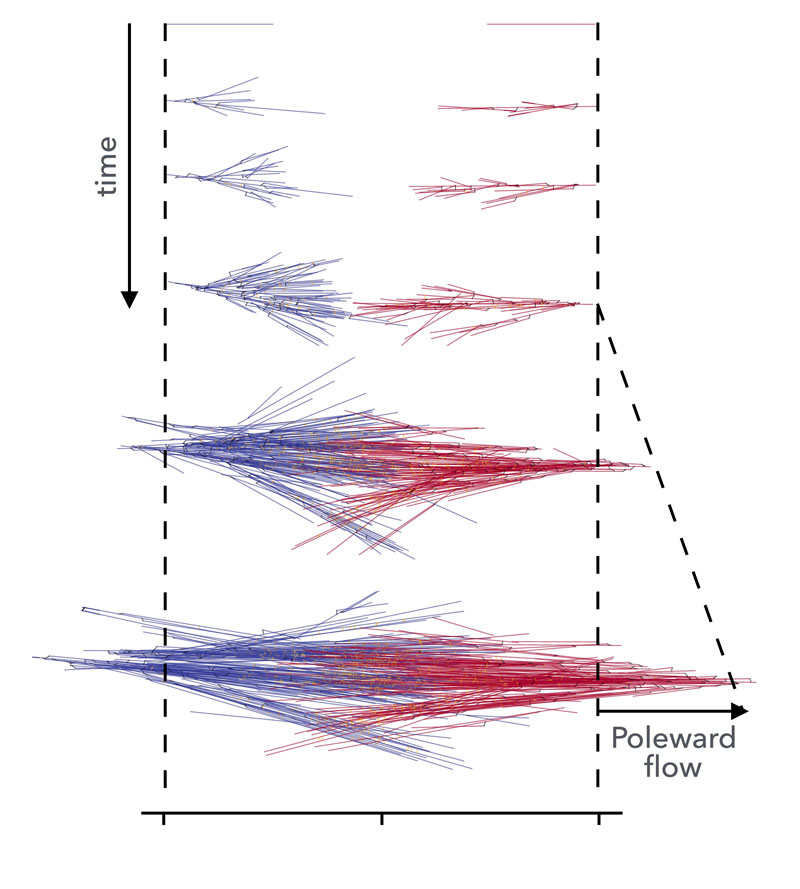
Figure: Simulation of bipolar spindle formation: two branching microtubule networks with opposite polarity collide, creating a region of mixed filament polarity. Molecular motors gelate the structure, coupling the two networks and actively driving them backward. This results in active poleward flows, consistent with those observed in Xenopus Laevis spindles.
The team of the HFSP Career Development awardee Dr. Jan Brugués (Max Planck Institute of Molecular Cell Biology and Genetics, Max Planck Institute for the Physics of Complex Systems and Center for Systems Biology Dresden, Germany) in collaboration with Dr. Frank Jülicher; recently published a study in Nature Physics [Dalton, Oriola, Decker, Julicher, Brugués, 2022] where they investigated how active flows are generated in the spindle, revealing an unexpected role of the spindle material properties on its internal architecture. Spindles were formed in a test tube using egg extracts from the African clawed frog Xenopus laevis. This method allows the generation of spindles in a cell-free environment, providing excellent imaging conditions and allowing for physical and biochemical perturbations. By combining femtosecond laser ablation and single molecule imaging, the researchers showed that active stresses in the spindle are generated by motor proteins in the central region of the spindle, and propagate outwards, i.e., towards the poles. Large-scale computer simulations of the active microtubule flows showed that this is consistent with the spindle being in a gel phase, where stresses can propagate over long distances, in this case distances spanning the whole structure. Surprisingly, computer simulations predicted that the architectural arrangement of microtubules in the spindle would reverse as a function of motor activity and the degree of gelation. The polarity reversal phenomenon arose as a consequence of the competition between branching microtubule nucleation and the poleward flux. This prediction was tested experimentally and spindles were found to reverse their polarity upon inhibition of the poleward flux.
In all, this investigation describes for the first time the full complex interplay between microtubule nucleation, active microtubule flows, and bipolar spindle architecture. A consistent model, uniting these seemingly disparate features, had eluded the field for many years. It is important to point out that such an understanding was only made possible by bringing together collaborators with a range of backgrounds, emphasizing the importance of interdisciplinary research when studying complex bio-molecular systems. For example, computer simulations, which are often used to complement experimental results, were used here to disentangle key phenomena which would have otherwise remained inaccessible. As such, the integration of detailed computer simulation into the project enabled greater exploration and provided a level of verification to the central arguments that would not have been possible with the experiments alone. Thus, in addition to solving a long outstanding problem in the field of active cytoskeletal self-organization by describing a well-known, essential, bio-molecular organelle from a perspective of complex materials physics, Dr. Brugués and collaborators have proven the importance of combined interdisciplinary research in addressing the complexities of living systems.
|
HFSP award information Career Development Award (CDA00074/2014-C): Mechanisms of spindle morphology and scaling during early development Awardee: Jan Brugués |


































