Ferroptosis is a novel, yet only partly understood, type of regulated necrotic cell death that may underlie some forms of degenerative diseases including neurodegeneration but that could also be harnessed to treat certain cancers. Oxidation of lipids in cellular membranes has been considered to be a central process in ferroptosis, yet the nature and source of lipid peroxidation has remained enigmatic. Two recent complementary studies from a 2014 HFSP Program Grant team, led by Marcus Conrad, demonstrated that ACSL4 (acetyl-CoA synthetase long chain family member 4), an enzyme involved in the activation of poly-unsaturated fatty acids (also frequently called PUFAs), provides the substrates for the generation of lethal lipid signals, which they identified to be arachidonic and adrenic acids incorporated into a specific class of phospholipids, i.e. phosphatidylethanolamines. Findings obtained in these studies will not only aid better understanding of the molecular mechanisms of this cell death pathway but will also provide novel cues in designing therapeutic strategies against ferroptosis-related diseases and in triggering cancer entities expressing ACSL4.
 |
Dr. Marcus Conrad, PhD, is a molecular biologist and group leader at the Institute of Developmental Genetics, Helmholtz Zentrum München, Germany. Long before the term “ferroptosis” was coined, he and his team developed several transgenic cell and mouse models for the study of the molecular mechanisms underlying ferroptotic cell death. In addition, his group has discovered the first class of in vivo efficacious ferroptosis inhibitors, called Liproxtstatins, that are currently being developed for the treatment of degenerative diseases with a ferroptotic signature. He is the coordinator of the HFSP Program Grant “Oxidized lipidome: the unspoken language of non-apoptotic cell death”. |
| Dr. Valerian E. Kagan is a biochemist and biophysicist (MV Lomonosov Moscow University, Russia) and is a Professor and Director of the Center for Free Radical and Antioxidant Health, University of Pittsburgh, PA, USA. His lab has focused on redox lipidomics as it relates to the signaling pathways in different types of cell death. His group has identified oxygenated cardiolipins as mitochondrial signals of apoptosis and hydroperoxy-phosphatodylethanolamines as ferroptotic signals. | |
 |
Dr. Judith Klein-Seetharaman is a Professor of Biomedicine and Systems Biology at the University of Warwick Medical School, UK. She was the founding co-director, with Raj Reddy, of the Biological Language Modeling Project at Carnegie Mellon University exploring the analogy that protein sequences are to their functions as words to meaning. The practical implication is that computational techniques developed originally for language can be directly applied to biological sequences. She has integrated computational and experimental approaches ever since and received prestigious prizes for her work from the Humboldt Foundation, Gates Foundation, NSF CAREER, Margaret Oakley Dayhoff Award of the Biophysical Society and most recently the EU Marie Curie International Incoming Fellowship. |
Cell death, despite the bad press, is an essential and daily occurring event in multicellular organisms’ lives. For instance, deliberate cell death induced by the body’s immune system is required to remove potentially pro-cancerous cells in order to reduce the risk of developing cancer. It is estimated that during a period of 24 hours in a human body more than 50 billion cells are lost due to the process of cell death. On the other hand, massive or premature cell death is the underlying cause of most (and many age-related) degenerative diseases including neurodegeneration as evident, for instance, in patients suffering Alzheimer’s and Parkinson’s diseases as well as amyotrophic lateral sclerosis (ALS).
The first identified and probably best understood form of cell death is apoptosis (from Ancient Greek: “falling off”). As early as 1842, the German scientist Karl Vogt was the first to describe the principle of apoptosis. The term apoptosis was eventually coined in a seminal work published in 1972 by Kerr and colleagues. A number of molecules that drive this kind of death were initially studied in the nematode Caenorhabditis elegans, and later shown to be of general significance to most multicellular organisms. While apoptosis is of utmost importance for both embryonic development of many multicellular organisms including man and deliberate clearance of aberrant cells by the immune cells later in life, necrotic cell death (i.e. necrosis) was originally and for a long time regarded to be an accidental and explosive form of cell death. This would make strategies with the goal to halt this form of cell death impossible. Intense research in the last decade, however, has unveiled that necrotic cell death is also regulated in many cases and can be subdivided into different cell death routines, each with distinctive molecular initiation mechanisms and execution pathways. Among approximately a handful of these regulated necrotic forms of cell death is ferroptosis (the term was first coined in 2012). Ferroptosis is characterized by iron-dependent lipid peroxidation (hence its name) and is entirely discernable from many other cell death pathways. Lipid peroxidation occurs when essential constituents of membranes, the so-called poly-unsaturated fatty acid residues, become oxidized and are not detoxified by glutathione peroxidase 4 (GPX4), a highly efficient enzyme specialized in removing these membrane-integrated peroxides. For this crucial function, GPX4 is now recognized as the key enzyme controlling ferroptosis. Uncontrolled lipid peroxidation, for instance by a malfunction (i.e. inhibition) or absence of GPX4, would otherwise cause ferroptotic cell death through still unknown cellular processes.
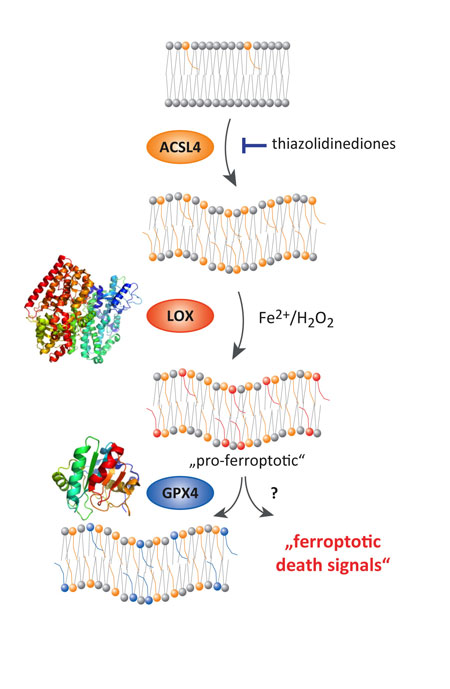
Figure 1
To shed light onto the molecular events causing lipid peroxidation and death, three research groups (Conrad, Kagan and Klein-Seetharaman) funded by an HFSP Research Grant have applied a multi-disciplinary approach. By using a number of different technologies including cellular, genetic, computational and pharmacological tools, as well as mass spectrometry analysis and modeling of cells and tissues undergoing ferroptosis, they identified ACSL4 as an essential regulator in ferroptosis. Its importance for ferroptosis was shown to rely on its ability to activate preferentially long chain, polyunsaturated fatty acids, which can normally fulfill many different functions in cells, such as energy supply, cell-cell communication and integral constituents of membranes which surround cells and organelles (Fig. 1). In the context of ferroptosis, however, the resulting activated fatty acid-CoAs need to be first incorporated into a specific class of phospholipids, which are the central components of any membrane in cells. Phospholipids are molecules that consist of two chains of fatty acids and one molecule of phosphate attached to glycerol. The phosphate group can be modified with simple organic molecules, such as choline or ethanolamine. Due to their chemical properties, phospholipids form lipid bilayers, which are essential to delimit a cell and its organelles, such as mitochondria and the endoplasmic reticulum. As phospholipids can vary dramatically in their composition because of the different molecules attached to the glycerol/phosphate group and the different fatty acids (e.g. number of carbon atoms = length, degree of double bonds = unsaturation) there might be thousands or even more than hundreds of thousands of different phospholipids in cells, making their analysis highly complex and extremely difficult. Yet we now show by using so-called redox-lipidomic analysis (an analytical approach to look for oxidized phospholipid species), bioinformatics and computer modeling that only a few phospholipids (i.e. phosphatidylethanolamines) having either arachidonic acid or adrenic acid, fatty acids with 20 or 22 carbon atoms and 4 double bonds, respectively, are the preferred oxidation substrates that accumulate during ferroptosis. This is highly intriguing as this oxidation appears to be very specific to these phospholipids and is not just a mere random oxidation of all phospholipids in the cell. Oxidation of these fatty acids is mediated by another class of enzymes, called lipoxygenases, generating free radicals and causing lipid peroxidation. If not removed by GPX4, lipid peroxides accumulate and can, in turn, be targets for iron dependent degradation leading to more lipid peroxidation and formation of toxic breakdown products.
Our study also suggests an intriguing function for vitamin E and closely related molecules as it is proposed that they are not only excellent general antioxidants in membranes but could directly and specifically inhibit lipoxygenases. Moreover, when the ACSL4 enzyme was inhibited by certain small molecules, including triacsin C and the antidiabetic class of compounds glitazones, or it is genetically inactivated in cells, cells became highly resistant to ferroptosis elicited either by ferroptosis-inducing molecules or by the genetic deletion of GPX4. This resistance mediated by ACSL4 was highly specific to this enzyme and unforeseen, as no other enzyme, when inactivated, has yielded such a strong protective effect against ferroptosis. Therefore, inhibiting ACSL4 by novel and specific pharmacotherapeutic agents might represent a highly efficient strategy in the context of pathologies, where premature cell death occurs, such as in neurodegenerative disease, organ transplantation, kidney and liver poisoning, etc.
Additional studies performed by our groups further revealed that the presence (i.e. expression) of ACSL4 in certain different breast cancer cell lines determines their sensitivity towards ferroptosis susceptibility. This was found to be particularly valid for tumor cells which are hard to treat by standard chemotherapy like the so-called triple-negative breast cancer cells (Fig.2). Treatment of these cells with ferroptosis-inducing agents effectively killed the cells in contrast to breast cancer cells which do not express ACSL4. This is a highly remarkable finding as the expression of ACSL4 in tumor biopsies, prior to treatment of patients with chemotherapy, may be used in future patient stratification when making the decision which sort of treatment regimen has the potential to efficiently eliminate the tumor in the body.
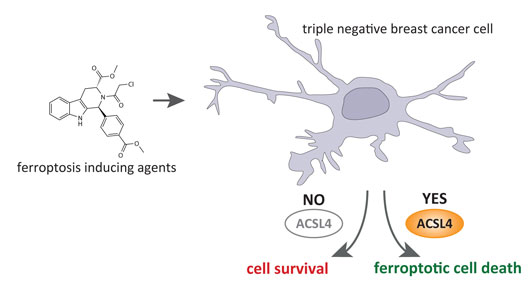
Figure 2
Conclusively, our discoveries presented in both publications provide previously unrecognized insights into the mechanistic role of specific lipid peroxidation and the essential players involved in ferroptotic cell death. Yet future studies are still warranted to further explain how specific peroxides in lipids indeed cause cell destruction and death and what the suspected role of iron in this context is. Yet, the implications of the findings presented here are manifold. For instance, the recognition that a small set of oxidized phospholipids steers the ferroptotic death process might be exploited in terms of its potential to be used as a marker (i.e. biomarker) for certain ferroptosis-related degenerative diseases. Considering the potential to pharmacologically manipulate ferroptosis by blocking ACSL4 might present a valuable target for novel therapies. Notably, existing and yet to be developed ACSL4-specific inhibitors might be used in the context of disease conditions, where the early and massive loss of cells causes organ dysfunction and even death. This might be particularly relevant for neurodegenerative diseases, such as Alzheimer’s, Huntington’s and amyotrophic lateral sclerosis (ALS), where neuronal cell loss eventually causes massive brain damage and death of affected patients. In fact, a recently performed meta-analysis of patients in Germany treated for more than two years with the type II antidiabetic drug pioglitazone (a glitazone which also inhibits ACSL4 as a side effect) highlighted that patients receiving such medication had a 47% reduced risk of developing dementia relative to nondiabetic patients. Whether this is due to the expected antidiabetic action of pioglitazone or due to its inhibiting effect towards ACSL4 certainly deserves further studies. Finally, the finding that the presence of ACSL4 determines the outcome of chemotherapeutic strategies to eradicate cancer cell growth is highly intriguing.
Hence, ACSL4 expression analysis of tumor biopsies might aid in deciding what kind of treatment has the potential to be productive and yield the desired outcome in patients suffering from cancer, thereby avoiding treating cancer patients with little or even non-effective chemotherapies. While being found to be true for a series of breast cancer cell lines, our findings on ACSL4 provide the rationale to further interrogate whether this holds true for other, still barely treatable tumor entities including pancreatic cancer, liver cancer, ovarian cancer and glioblastoma.


































